Lung volume augmentation
A spinal cord injury (SCI) with a neurological level of injury (NLI) above T12 will result in some degree of respiratory function change. Early assessment will determine predictive factors for respiratory function changes and complications. Frequent monitoring will determine the adequacy of ventilation and sputum clearance, as well as any deterioration in breathing patterns and increase in the work of breathing.
Outcomes will ultimately determine ongoing ventilation and respiratory health needs, depending on the extent of chronic respiratory dysfunction and persistent respiratory risk factors.
Respiratory changes
Respiratory changes
There are numerous aspects to respiratory function change following an acute spinal cord injury (SCI).
These may include:
- respiratory neuromuscular weakness involving the intercostals, abdominals and possibly even the diaphragm
- reduced diaphragm efficiency due to altered chest wall and abdominal muscle tone and compliance
- restricted inspiratory capacity and decreased forced expiratory volumes and flow rates
- impaired sigh, cough and other forced expiratory actions
- atelectasis and sputum retention
- hypoventilation, which may progress to hypoxaemia and hypercapnia
- increased work of breathing due to altered breathing mechanics—especially in sitting
- increased risk of respiratory fatigue and failure, as well as sleep-disordered breathing.
In summary, these changes collectively lead to reduced ventilation and secretion clearance, along with an increased work of breathing.
For an acute, motor-complete NLI at T6 or above, but especially at C5 and above, significant respiratory function changes occur due to neuromuscular weakness and paralysis, compounded by the presence of spinal shock, as well as autonomic nervous system (ANS) disruption. Key respiratory muscles affected include the intercostal, abdominal and diaphragm muscles.
At this time, weakened or paralysed respiratory muscles present with flaccidity and increased compliance due to spinal shock. This affects diaphragm positioning and function, as well as breathing mechanics. Ultimately, this results in reduced ventilation and secretion clearance. Paradoxical breathing can also develop, which is very inefficient and increases the work of breathing.
For more information on respiratory function changes following SCI, refer to Respiratory changes.
Without adequate and timely intervention, the combination of hypoxaemia, hypercapnia and respiratory fatigue is a key precursor to the onset of respiratory failure. In addition to this, chronic hypoventilation also contributes to sleep-disordered breathing, compounded by any other obstructive and central factors.
During the acute management phase, the following key interventions should be implemented to optimise respiratory management:
• ventilation support
• lung volume augmentation
• secretion management.
These interventions are complementary and should be tailored to the person with SCI’s individual needs. While both ventilation and lung volume augmentation interventions may involve similar elements—such as patient positioning and the use of positive pressure breathing—their roles and application differ. The distinction is defined as follows.
Ventilation support includes use of positioning and mechanical ventilator devices—often continuously—to normalise gas exchange, stabilise the airway, and reduce the overall work of breathing.
Lung volume augmentation includes a range of respiratory techniques and devices—typically in short, repeated treatment sessions—to achieve the therapeutic benefits of deep breathing, support secretion clearance, and promote respiratory muscle conditioning.
The role of lung volume augmentation
Following a high-level SCI, significant hypoventilation may develop due to respiratory muscle weakness and paralysis, affecting the ability to generate normal tidal volumes and access inspiratory reserve volumes. Expiratory volumes and flow rates are also reduced, impacting secretion clearance. Altered breathing patterns develop, as well an increased work of breathing. The combined outcome of hypoventilation is an increased risk of sputum retention, respiratory fatigue and ultimately respiratory failure.
Positive-pressure breathing via mechanical ventilation is utilised to improve gas exchange, reduce the work of breathing and manage the risk of respiratory complications. Lung volume augmentation can be used to supplement this as described below.
The primary goals of lung volume augmentation are to:
- generate high-volume inspiration (beyond the capacity of weakened respiratory muscles and typical mechanical ventilation tidal volumes, breathing into inspiratory reserve volumes), to achieve the benefits of deep breathing
- inflating collapsed airways and alveoli
- stimulating greater surfactant production, further improving alveolar compliance and reducing airway resistance
- increasing lung and chest wall compliance- with caution
- enhancing forced vital capacity (FVC) and peak cough flow (PCF)
- mobilising secretions, to further address atelectasis and reduce the risk of pneumonia
- helping to prevent respiratory fatigue associated with treatment
- improve respiratory muscle strength, to achieve voluntary higher tidal and inspiratory reserve volumes to support
- the process of weaning from ventilation supports
- coughing for self-management of secretion clearance
- progression to sitting, engagement in rehabilitation and activities of daily living (ADLs)
- improvements in speech.
With appropriate medical clearance, lung volume augmentation techniques and devices for a person with SCI should be considered as indicated.
Treatment should be delivered by a physiotherapist, or by clinicians and carers, who have been adequately trained by a physiotherapist.
When using lung volume augmentation techniques and devices, the overall approach is to introduce deeper breathing —beyond normal tidal volume, into inspiratory reserve volume—while minimising treatment-related fatigue for a person with SCI.
To support safe and effective delivery, sessions should be conducted efficiently by:
- timing with post bronchodilator and pain relief administration
- managing the energy demand of the treatment
- clustering sessions with personal care tasks to allow deliberate rest periods afterwards.
Techniques and devices may be used in combination, along with secretion management, unless specifically contraindicated or not tolerated. In some cases, lung volume augmentation may need to be repeated at the end of a treatment session, to reverse cough-induced atelectasis. This addresses any airway collapse and re-establishes the functional residual capacity of the lungs for ongoing ventilation.
Types of lung volume augmentation
An overview of lung volume augmentation techniques and devices are provided below, including references to recommendations from The Australian and New Zealand Clinical Practice Guidelines: For the Physiotherapy Management of People with Spinal Cord Injury.
The Australian and New Zealand Clinical Practice Guidelines: For the Physiotherapy Management of People with Spinal Cord Injury
Many lung volume augmentation techniques are clinically practised today by physiotherapists. They have evolved over decades, in the treatment of post-surgical and neuromuscular conditions, including SCI. While there is considerable clinical experience in the use of these interventions, there has been limited scientific study of their efficacy in the management of SCI respiratory dysfunction.
In 2022, The Australian and New Zealand Clinical Practice Guidelines: For Physiotherapy Management of People with Spinal Cord Injury were developed, providing evidence-based recommendations where possible, or consensus-based opinion statements.
The process completed systematic reviews and SCI professional peer consultation to address physiotherapy enquiry questions, presented in a PICO (Participant, Intervention, Comparison and Outcome) format. The outcomes are presented as either evidence or consensus statements and form the new clinical practice guidelines. Those which are relevant to the management of respiratory function following SCI, have been incorporated into the “Lung Volume Augmentation” and “Secretion Management” sections. They will be presented using the following colour variations banners.
The Australian and New Zealand Physiotherapy Guidelines for people with SCI states:
Evidence: Weak for recommendation
Guideline
The Australian and New Zealand Physiotherapy Guidelines for people with SCI states:
Consensus: Weak for recommendation
The Australian and New Zealand Physiotherapy Guidelines for people with SCI states:
Consensus: Strong for recommendation
Guideline
The Australian and New Zealand Physiotherapy Guidelines for people with SCI states:
Consensus: Weak against recommendation
Guideline
Supine positioning
The Australian and New Zealand Physiotherapy Guidelines for people with SCI states:
Consensus: Strong for recommendation
Positioning in supine should be provided (in favour of sitting) to improve lung volumes in people with SCI who have abdominal muscle paralysis or weakness.
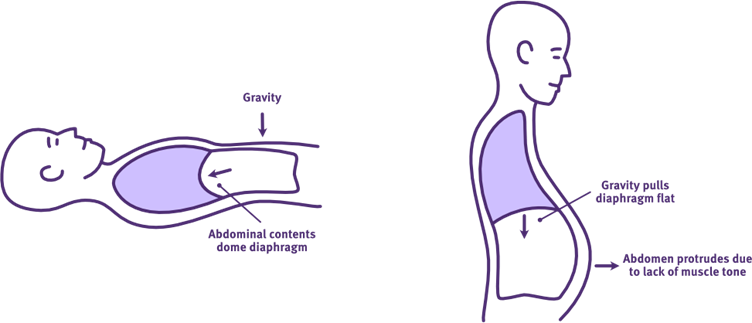
The effect of positioning on diaphragm function following acute high-level SCI
Adapted from Thoracic Key
While mechanical ventilation is often required following SCI, supine positioning is also another important “first-response” and management consideration. This is not only because spinal precautions may be required, but because supine positioning will complement mechanical ventilation to improve respiratory function.
Supine positioning is recommended during acute respiratory management, as it has an immediate and passive influence on ventilation and lung volumes following SCI. It uses gravity to address the negative effects of abdominal muscle weakness, flaccidity and increased abdominal wall compliance, on diaphragm positioning and function. Gravity helps to position the abdominal contents against the diaphragm, improving its dome-shape at rest and excursion range during inspiration. This improvement in its length-tension relationship for contraction, enhances the efficiency of diaphragm function, improving tidal volumes and reducing the work of breathing.
While supine positioning is optimal, sometimes positioning changes are required for other aspects of SCI management and care e.g. skin breakdown, aspiration emergency. Use of a high-quality, pressure relief mattress may permit prolonged supine positioning. Otherwise, any changes in positioning should be closely monitored for potential negative effects on ventilation and the work of breathing.
Supine may not be suitable for people with significant abdominal distension, central adiposity or those with large abdomens and long-standing SCI.
Sitting is not recommended during acute respiratory management. In the presence of excessive abdominal wall compliance, gravity allows the abdominal contents to fall away from the diaphragm, flattening its dome-shape at rest. This limits the efficiency of diaphragm function during inspiration, worsening tidal volumes and the work of breathing.
When to introduce sitting
Beyond the acute management phase after a SCI, any respiratory muscle recovery and strengthening which occurs due to neural plasticity and rehabilitation will ultimately enhance respiratory function. As spinal shock resolves over weeks to months, intercostal and abdominal muscle flaccidity may transition to spasticity. Combined with any chronic chest wall immobility, the tendons, ligaments and joints of the rib cage stiffen.
This typically:
- reduces excessive chest wall and abdominal compliance
- helps correct a paradoxical breathing pattern
- decreases the work of breathing.
Typically, there is also some partial rebalancing of ANS disruption and a reduction in vagal overactivity. Negative cardiovascular and respiratory effects slightly diminish, and respiratory function improves further.
With these adjustments, weaning from ventilation supports and progression to sitting may be introduced. However, weaning from ventilation support and the introduction of graduated sitting should be carefully planned by the multidisciplinary team, with appropriate timing and staging to promote success.
Sitting in bed: limit
Inclining the bedhead introduces gravitational load, increasing the ventilatory demand on the diaphragm to maintain tidal volumes—this increases the work of breathing. An additional and significant concern is the impact of prolonged upright bed positioning on skin integrity.
Sitting in a reclining or tilt wheelchair with supports
The preferred method for introducing sitting is to use a recliner or tilt-in-space wheelchair, with an appropriate pressure-relieving cushion, headrest, and if required, elevating leg rests. These wheelchairs enable a controlled sitting orientation between supine and upright sitting, for immediate repositioning if necessary e.g. hypotensive episode.
This approach is particularly useful to:
- manage the initial risk of respiratory fatigue, due to increased ventilatory demand on the diaphragm
- manage the risk of hypotension, associated with ANS disruption
- optimise skin care for seating, as sitting time gradually increases.
Sitting in an upright wheelchair with supports
Once upright sitting is well tolerated for at least 6–8 hours, transition to a fully upright wheelchair can be considered. Adequate postural supports should be incorporated, including a solid backrest with appropriate height and contour, armrests and a chest strap to minimise the risk of falls.
These seating supports aim to:
- achieve postural symmetry at the pelvis and trunk
- promote thoracic extension with optimal head alignment
- assist sitting balance and upper limb function for activities of daily living (ADLs)
- optimise respiratory function, including periods of rest.
Use of an abdominal binder in sitting
The Australian and New Zealand Physiotherapy Guidelines for people with SCI states:
Evidence: Weak for recommendation
Abdominal binders in sitting may be provided to improve lung volume in people with SCI who have abdominal muscle weakness or paralysis.
If abdominal muscle paralysis or significant weakness is present, the use of an abdominal binder is recommended when sitting—and possibly standing.
A well-fitted abdominal binder helps counteract the loss of normal abdominal muscle tone and increased abdominal compliance, when changing position from supine (where gravity is providing positioning assistance). It applies pressure across the abdomen onto the abdominal contents to support:
- venous return in the inferior vena cava, to maintain blood pressure
- dome-shaping of the diaphragm, to improve its position for efficient contraction
- passive and forced expiration.
Overall, the abdominal binder is clinically used in the management of orthostatic hypotension, along with the use of compression stockings and pharmacological interventions.
Due to respiratory changes following SCI, the abdominal binder is also used to enhance lung volumes and reduce the work of breathing in sitting, while improving coughing. One study reported that use of an abdominal binder in sitting for people with a complete SCI above T1, improved maximal inspiratory pressure (MIP), vital capacity (VC), forced expiratory volume in 1 second (FEV1) and peak expiratory flow (PEF). It also increased the time for sustained voice.
Anecdotal evidence suggests that an abdominal binder may also assist with balance and postural cueing during early rehabilitation.
For more information about the prescription and application of an abdominal binder, refer to Abdominal binder.
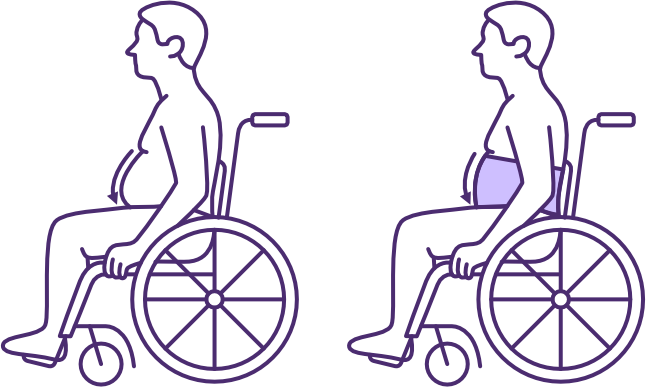
Effect of an abdominal binder on abdominal compliance in sitting following high-level SCI
Image source QSCIS
Positive-pressure breathing
Positive-pressure breathing devices are often required to achieve lung volume augmentation. This may be via a type of ventilator, or specific positive-pressure devices which provide single, larger volume breaths, or multiple, stacked volume breaths. The aim is to achieve the user’s maximal insufflation capacity (MIC) while significantly reducing the work of breathing associated with this.
As previously discussed, when spinal shock is present, supine positioning helps improves diaphragm positioning and function. Similar to mechanical ventilation, positive-pressure breathing devices also addresses the impact of intercostal muscle weakness, flaccidity and increased chest wall compliance during higher volume breathing. Together, these interventions are complementary, as both correct aspects of the paradoxical breathing pattern and reduce the work of breathing associated with this.
Positive-pressure breathing devices also require a circuit and airway interface, with most offering the options of mouthpieces or face masks. Some devices are appropriate for use with tracheostomy and endotracheal intubation, while others are not.
Specialist SCI units may have access to high-cost positive-pressure breathing devices. Generalist units may need to utilise low-cost options instead, but these are often very useful for maintaining respiratory health in the community, after hospital discharge.
When using positive-pressure deep breathing devices, considerations include:
- in the acute phase, when spinal shock is present, there is a mixed presentation of a flaccid chest wall +/- increased airway resistance from atelectasis, bronchospasm, secretion retention and any ARDS risk; hence, there is a need for cautious progression, starting with low pressures and volumes to reduce the risk of volutrauma/barotrauma
- in the chronic phase, following spinal shock resolution, there is a tendency for the chest wall, thoracic muscles and collapsed alveoli to progress to stiffness/spasticity and ankylosis/fibrosis; hence, this is a rationale for preventative maintenance; if beginning after a long time post-injury, again there is a need for cautious progression, starting with low pressure and volumes to reduce the risk of volutrauma/barotrauma
- selecting the appropriate treatment position– in the acute phase, the work of breathing is reduced in supine; alternate positioning may promote variations in lung perfusion and ventilation, as well as postural drainage
- selecting the appropriate techniques, with their associated devices– these are discussed in more detail below
- selecting the appropriate interfaces and circuits– either mouthpiece or face mask along with compatible circuits with or without an exhalation valve/leak port; sometimes endotracheal and tracheostomy connectors may be an option, but have unique considerations with respect to cuff inflation or glottal function
- determining a safe inspiratory positive airway pressure (IPAP)/inspiratory pressure (PI) and/or inspiratory reserve volume (IRV) to achieve lung volume augmentation, while reducing the risk of volutrauma/barotrauma; the incorporation of expiratory positive airway pressure (EPAP) may assist further, if available
- completing subjective assessments to adjust device parameters for age and size, determine user tolerance, adequacy of chest wall expansion during inspiration, and the effectiveness of cough or voice following insufflation
- completing objective assessments such as observation, palpation and spirometry to evaluate efficacy, particularly for forced vital capacity (FVC) as an indicator of vital capacity (VC), as well as peak cough flow (PCF) to monitor the effectiveness of coughing.
The following factors prohibit the safe use of positive-pressure breathing devices:
- reduced airway protection, including bulbar dysfunction and any increased aspiration risk
- poorly controlled intracranial pressures
- severe facial injuries
- haemodynamic instability, including arrhythmias, neurogenic shock, pulmonary embolism and autonomic dysreflexia
- abdominal distension, including paralytic ileus or recent abdominal surgery
- thoracic complications, including undrained pneumothorax, tracheoesophageal fistula, haemoptysis, adult respiratory distress syndrome (ARDS)
- significant chronic obstructive pulmonary disease (COPD).
Non-invasive ventilation (NIV)
The Australian and New Zealand Physiotherapy Guidelines for people with SCI states:
Consensus: Strong for recommendation
Intermittent application of positive pressure devices should be provided to improve lung volume in non-ventilated people with acute SCI who have respiratory muscle weakness.
Positive pressure devices include Continuous Positive Airway Pressure (CPAP) and brief periods of Bilevel Positive Airway Pressure (BiPAP).
Non-invasive ventilation (NIV) is typically used to provide ventilation support, synchronised with the respiratory cycle of a user who is spontaneously breathing. However, it can also be used to improve access to inspiratory reserve volumes and reduce the associated work of this breathing. NIV devices may provide either single (pressure-cycled NIV) or stacked breaths (volume-cycled NIV).
NIV devices can provide expiratory positive airway pressure (EPAP) support to minimise airway collapse during exhalation, which improves functional residual capacity and therefore lung compliance. Some NIV devices also permit setting of an inspiratory airway pressure (IPAP) which can be used to augment inspiratory tidal volumes. Other than enhancing inspiratory volume to improve a voluntary cough, NIV devices do not provide any exsufflation.
Some types of mechanical insufflation-exsufflation (MI-E) devices also offer a burst NIV mode (not for users with a tracheostomy or ventilator dependent). However, this requires a suitable circuit inclusive of an exhalation valve/leak port, instead of the standard MI-E circuit and is time limited e.g. <15 minutes.
For more information, refer to Ventilation support: Non-invasive ventilation and Lung Volume Augmentation: MI-E below.
Intermittent positive-pressure breathing (IPPB)
The Australian and New Zealand Physiotherapy Guidelines for people with SCI states:
Consensus: Strong for recommendation
Intermittent application of positive pressure devices should be provided to improve lung volume in non-ventilated people with acute SCI who have respiratory muscle weakness.
Positive pressure devices include Intermittent Positive Pressure Breathing (IPPB).
Historically, intermittent positive-pressure breathing (IPPB) has been used in the treatment of various respiratory conditions, including post-surgical recovery and neuromuscular impairments such as SCI. From the late 1950s, the Bird Mark 7 respirator/ventilator became a widely used IPPB device. Although discontinued in the late 1980s, references to the Bird device remain common in scientific literature.
While IPPB therapy is now less commonly used, newer versions of IPPB devices are available and may still play a role in treatment for a person with SCI who is spontaneously breathing. Some types of mechanical insufflation-exsufflation (MI-E) devices also offer a IPPB mode. However, this requires a suitable circuit inclusive of an exhalation valve/leak port to be used, instead of the standard MI-E circuit.
IPPB therapy requires the user to trigger the positive-pressure breath and cooperate with the technique. It can be used to achieve maximal insufflation capacity (MIC) and reduce the associated work of this breathing. IPPB devices provide only single (pressure-cycled) breaths. However, IPPB devices do not provide any expiratory positive airway pressure (EPAP) during exhalation (although some devices may be able to provide some expiratory resistance). Other than enhancing inspiratory volume to improve a voluntary cough, IPPB devices do not provide any exsufflation.
Pre-programming IPPB settings allows standardised lung volume augmentation to be delivered across a 24-hour period by trained non-physiotherapy staff, including nurses, carers, and family members. However, training is essential to ensure safe and effective delivery.
If appropriate, IPPB provides the benefits of intermittent lung volume augmentation, while reducing the work of breathing required. However, repeated or ineffective coughing efforts may contribute to respiratory fatigue over time during the acute management phase. For these reasons, mechanical insufflation–exsufflation (MI-E) devices are increasingly used as a preferred device for lung volume augmentation, as the work of breathing for secretion management is also reduced.
IPPB method
- An IPPB device uses compressed air +/- oxygen and is typically flow-controlled, but pressure-cycled.
- The operator sets the maximum inspiratory pressure, flow rate, and inspiratory trigger sensitivity.
- The user initiates a spontaneous breath by generating negative inspiratory pressure against the IPPB interface via an appropriate circuit and interface attached to the user. Device sensitivity can be further adjusted to better detect the user’s effort, and flow rate can be tuned to match the user’s breathing pattern.
- A positive-pressure breath is delivered by the device (typically starting at 10–15 cmH₂O, increasing up to a maximum of 40cmH₂O), supporting lung volume augmentation with minimal effort from the user.
- Expiration occurs passively through the attached circuit inclusive of an exhalation valve/leak port.
- The user completes a pre-determined series of IPPB-assisted breaths.
- Following the final breath, a manual assisted cough (MAC) can be applied to facilitate a strong cough. This requires coordinated effort between the user and the operator.
IPPB variations
Additional features include:
- attachment options such as a non-vented mask, mouthpiece, or tracheostomy connector
- aerosolised medication, including saline
- ambient air or supplemental oxygen via an air-mix system
- parameter variations such as inspiratory pressure, flow rate and trigger sensitivity.
Recommendations for use include:
- a mouthpiece is often more comfortable and successful than a mask for triggering inspiration
- an increase in maximum inspiratory pressure and a decrease in flow rate will promote a longer and deeper breath, and vice versa
- IPPB may be an option for ventilated users with tracheostomy who are conscious, medically stable and are assessed to be ready for weaning, but extreme caution is required to reduce the risk of volutrauma/barotrauma. In these cases:
- it may be safer to leave the invasive airway cuff deflated and permit some leakage, rather than inflating to maximise pressure delivery to the lung periphery
- inspiratory pressure settings may need to be carefully increased to account for increased airway resistance, dead space and leakage.
IPPB more information
More information about IPPB can be found here.
Mechanical insufflation-exsufflation (MI-E)
The Australian and New Zealand Physiotherapy Guidelines for people with SCI states:
Consensus: Strong for recommendation
Intermittent application of positive pressure therapy techniques should be used (in consultation with medical staff) for improving lung volume in ventilated people with acute SCI that are medically stable.
Positive pressure therapy techniques include mechanical insufflation.
Intermittent application of positive pressure devices should be provided to improve lung volume in non-ventilated people with acute SCI who have respiratory muscle weakness.
Positive pressure devices include mechanical insufflation.
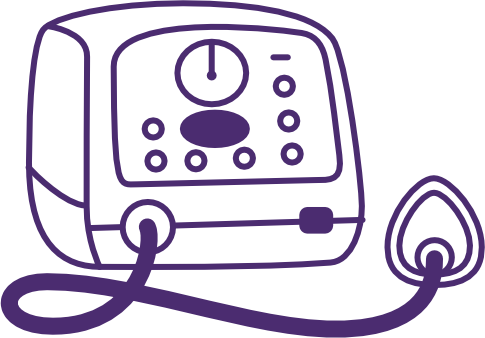
Mechanical Insufflation-Exsufflation (MI-E) device
Adapted from SCIRE Professional
Although first developed during the polio epidemic, mechanical insufflation-exsufflation (MI-E) technology has since evolved and is now commonly referred to as a “Cough Assist” device.
MI-E devices provide both lung volume augmentation (insufflation = inspiration) and cough augmentation (exsufflation = forced expiration). These two modes are complementary—by increasing inspiratory volume, expiratory flow and volume are also enhanced, leading to improved cough effectiveness.
MI-E therapy does not require the user to be able to breathe spontaneously or cooperate, so it can be cautiously introduced with invasive ventilation. However, if available, use of any ventilator hyperinflation setting is initially preferable to reduce the risk of volutrauma/barotrauma before introducing MI-E therapy. However, MI-E therapy is certainly advantageous to introduce prior to, during and after any weaning for extubation/decannulation.
MI-E devices may provide single breaths, with some also permitting stepped insufflations. Either way, it can be used to achieve maximal insufflation capacity (MIC) and reduce the associated work of this breathing. While most devices do not provide expiratory positive airway pressure (EPAP) during exhalation, all MI-E devices provide exsufflation.
Pre-programming MI-E treatment sessions allows lung volume and cough augmentation to be standardised and delivered throughout a 24-hour period by trained non-physiotherapy staff, such as nursing staff, carers, or family members. However, specific training is essential to ensure safe and effective use.
Using a single device, MI-E therapy enables both lung volume and cough augmentation, with their associated respiratory benefits. As the work of breathing for both is significantly reduced, treatment is less likely to contribute to respiratory fatigue during acute management.
MI-E method
- The MI-E device uses compressed air +/- oxygen and is typically pressure-controlled, but time-cycled.
- The operator sets various parameters including whether treatment will be insufflation +/- exsufflation, as well as the maximum pressures and time for insufflation +/- exsufflation, the insufflation flow rate or rise time and inspiratory trigger sensitivity.
- The operator initiates mechanical insufflation using the device toggle switch (on the device, hand remote or foot pedal) via an appropriate circuit and interface attached to the user.
- A positive pressure breath is delivered by the device (typically 20 to 40 cmH₂O), up to the user’s maximal insufflation capacity (MIC). This supports lung volume augmentation with minimal effort from the user.
- Expiration occurs passively through the attached circuit with nil exhalation valve/leak port (unless using the following other non-MI-E modes on the same device).
- A series of insufflation breaths is delivered for lung volume augmentation and sputum mobilisation; a mechanical assisted cough can then be triggered by rapidly reversing airflow to generate negative pressure (typically –20 to –40 cm H₂O). This sudden shift in pressure simulates a cough, without requiring active effort from the user. The effectiveness of the cough is enhanced by increasing the pressure gradient between insufflation and exsufflation, thereby increasing forced tidal volume and peak expiratory flow. A manual assisted cough (MAC) can be applied simultaneously to further improve cough effectiveness.
- While optimal MI-E dosage and frequency has not been determined yet, a typical MI-E treatment session may be repeated cycles of 3 pre-therapy breaths, 2 exsufflations, 2 post-therapy breaths. Other treatment approaches may consist of cycling between insufflation and exsufflation, with a exsufflation cough as needed.
MI-E variations
Modes may include:
- manual– allows the operator greater control over insufflation and exsufflation timing and pressures
- automatic– enables pre-programmed cycle for consistent, repeatable therapy—suitable for unsupervised use by trained clinicians and carers
- IPPB– supports programmed lung volume recruitment initiated by the user when needed
- burst NIV– provides EPAP typically used before or after treatment for respiratory rest, while maintaining any lung volume recruitment; it is not intended for continuous ventilatory support and will be time limited e.g. <15 minutes; it is not for users with a tracheostomy or endotracheal tube
- Intrapulmonary Percussive Ventilation (IPV) or “Percussor Mode”– provides airway percussion therapy with settings for frequency (e.g. 10-780 CPM), pressure (e.g. 0-70 cmH20) and I:E ratios (e.g. 1:5 to 5:1)
- High Frequency Chest Wall Oscillations (HFCWO)– typically using an inflatable chest wrap to provide external chest wall percussion with settings for frequency (e.g. 50-900 bpm), pressure (e.g. 5-70 cmH20) and I:E ratios (typically 1:1).
Circuit design must be appropriate to the mode, as well as the actual device.
- Most MI-E devices use a single lumen circuit, although dual lumen may be required, ensuring there is no contamination between insufflation and exsufflation debris
- Manual and automatic MI-E modes use standard MI-E circuits which have no exhalation valve/leak ports, to allow for delivery of negative pressure delivered in short treatment sessions (disconnect user from MI-E device when each short treatment session is complete)
- In contrast, IPPB, NIV and IPV modes require a circuit with an exhalation valve/leak port, to prevent CO₂ rebreathing during longer use (disconnect user from MI-E device when treatment is complete).
- Some circuits have the exhalation valve/leak port as an extra attachment (it is normally preferable to operate with 2 circuits: 1 circuit dedicated to MI-E and another circuit dedicated to the other modes)
- Some circuits have a switch valve on the circuit to open or close when changing between modes (MI-E mode (closed) and IPPB, NIV, IPV (open)).
Additional features may include:
- interface options such as a non-vented mask (preferred) versus mouthpiece, and sometimes a tracheostomy or endotracheal tube connector
- options for ambient air, and sometimes supplemental oxygen via an entrainment port and/or humidification
- recorded measures of volume and exsufflation flow rate, pulse oximetry for monitoring during treatment sessions
- real time pressure wave and bar graphs for feedback during treatment sessions
- real time capacity to record or adjust treatment sessions
- parameter variations may include
- insufflation and exsufflation pressures and time ratios, with some providing an option for stepped insufflation
- pause time between insufflation and exsufflation/cough
- flow rate or rise time options for each insufflation
- sensitivity trigger options for insufflation
- synchrony options for insufflation or exsufflation/cough
- airway oscillations on insufflation, exsufflation or both with a selection range for frequency (e.g. 4-20 Hz) and amplitude (e.g. 1-10 cmH20)
- EPAP on pause breath
- options for pre-therapy and post-recruitment breaths
- number of pre-save programs for different types of therapies, including when well and unwell.
Additional accessories may include:
- option of a stand as well as mask holders
- internal battery (e.g. 3-4 hr capacity) in addition to external power supply
- hand remote or foot pedal
- pulse oximeter.
Additional variations may include:
- overall weight of device
- device handle, carry bag etc.
- customisation of screen colours, or orientation of screen for visualisation
- touch screen versus button operation, including varying sensitivity and ease of navigation
- connectivity e.g. ethernet, USB, USB C, HDMI port, flash drive, wifi, bluetooth
- remote control access e.g. app to adjust device settings via a mobile phone
- data download processes i.e. simple vs multi-stage
- access to compliance summary/user log
- safety prompts about correct circuit +/- external valve/leak port
- terminology and use of positive (+) and negative (-) numbers for insufflation and exsufflation
- provision of user quick reference guides and online resources.
MI-E clinical reasoning
Recommendations for use include:
- during the initial use of MI-E carefully monitor oxygen and heart rate settings to ensure safety; if there is a high sputum load, it may be advisable to have suction and resuscitation equipment ready
- start pressure settings low and seek user feedback, while monitoring chest wall expansion to determine adequacy of pressure settings; typically start insufflation with 15 cmH20 and increase in 5 cmH20 increments (unless the user is also accessing NIV, then start insufflation NIV IPAP +5 cmH20 and increase in 5 cmH20 increments)
- keep exsufflation pressure 5-10 cmH20 more negative than insufflation pressure to maintain a pressure gradient that biases sputum mobilisation
- progressively increase pressures until efficacy is achieved, remembering the delivered tidal volume varies depending on airway resistance, lung compliance, and circuit integrity
- individually adjust inspiratory and expiratory time/rise/pressures for each user, matching their normal breathing pattern and optimising comfort
- if adding oscillations, adjust insufflation and exsufflations pressures to factor in the additional pressure overlay; however, there is little evidence this is a useful feature when compared to options with percussor modes
- if there are concerns about chest wall and spine bone mineral density, or chest wall and lung compliance, start with lower flow rates and pressures; also opt for IPV mode rather than use HFCWO
- measures of volume and peak flow rate are not always accurate and represent gas decompression in the device circuit, so also compare to pressure-wave forms as well as observations of the chest wall and sound of the cough effectiveness
- when the user is spontaneously breathing, use of a non-vented face mask is preferable to a mouthpiece
- MI-E use may be an option for ventilated users, but extreme caution is required to reduce the risk of volutrauma/barotrauma. In these cases:
- it may be safer to leave the invasive airway cuff deflated and permit some leakage, rather than inflating to maximise pressure delivery to the lung periphery
- inspiratory pressure settings may need to be carefully increased to account for increased airway resistance, dead space and leakage.
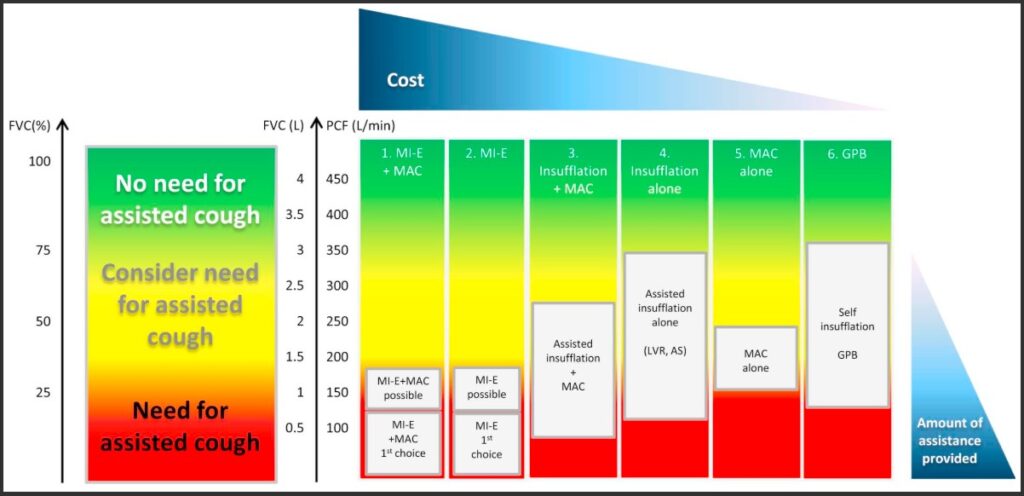
Graph of spirometry indicators for lung volume augmentation and cough augmentation in neuromuscular disorders
Toussaint, M., Chatwin, M., González, J., Berlowitz, D. J., & ENMC Respiratory Therapy Consortium. (2018). 228th ENMC International Workshop: Airway clearance techniques in neuromuscular disorders, Naarden, The Netherlands, 3–5 March, 2017. Neuromuscular Disorders, 28(3), 289–298. https://dhttps://doi.org/10.https://www.nmd-journal.com/article/S0960-8966(17)30588-6/fulltext#f00101016/j.nmd.2017.10.008
The following are examples of published flowcharts for the safe introduction and titration of basic MI-E parameters in adults with neuromuscular disease, including SCI. It is also important to note that device pressure and volume readings may not accurately indicate the lung volume augmentation or peak cough flow achieved. Rather subjective reports from the user and objective assessment of chest wall expansion and the sound of the cough should also inform titration.
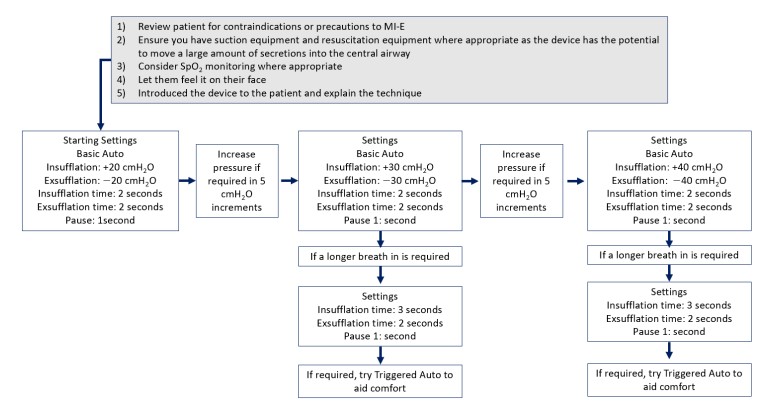
Flowchart for a standard approach to initiation of MI-E in adults
Chatwin, M., & Wakeman, R. H. (2023). Mechanical Insufflation-Exsufflation: Considerations for improving clinical practice. Journal of Clinical Medicine, 12(7), Article 2626. https://doi.org/10.3390/jcm12072626
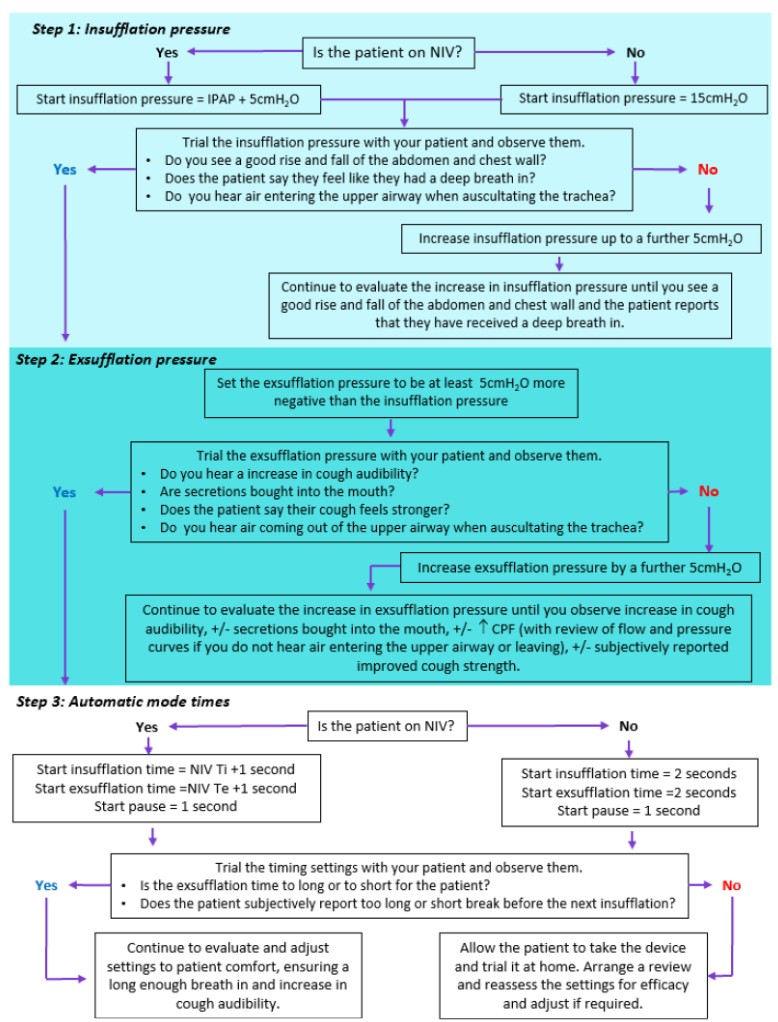
Flowchart for a personalised approach to titration of MI-E in adults
Chatwin, M., & Wakeman, R. H. (2023). Mechanical Insufflation-Exsufflation: Considerations for improving clinical practice. Journal of Clinical Medicine, 12(7), Article 2626. https://doi.org/10.3390/jcm12072626
MI-E video information
MI-E more information
More information about MI-E can be found here.
In addition to factors which prohibit the safe use of positive-pressure breathing devices, the following also prohibits the safe use of MI-E devices:
- drained pneumothorax- due to negative exsufflation pressures
- unconscious- due to lack of user feedback.
Lung volume recruitment (LVR)
The Australian and New Zealand Physiotherapy Guidelines for people with SCI states:
Consensus: Weak for recommendation
Air stacking may be taught to improve lung volume in people with SCI who have respiratory muscle weakness.
Air stacking involves the use of a positive pressure inspiratory device and should be used with a mouthpiece and nose peg, rather than a face mask—because of the risk of barotrauma/pneumothorax if a facemask is provided.
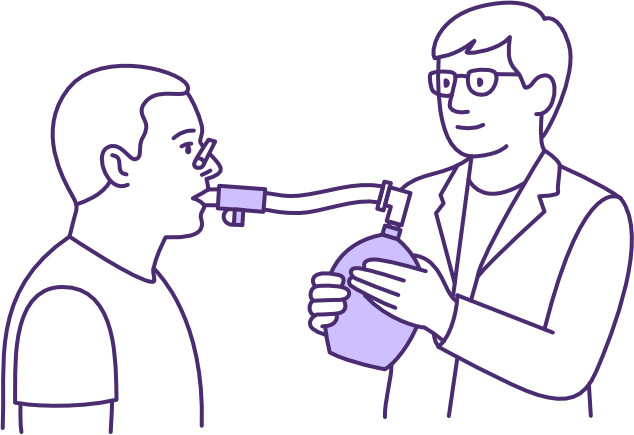
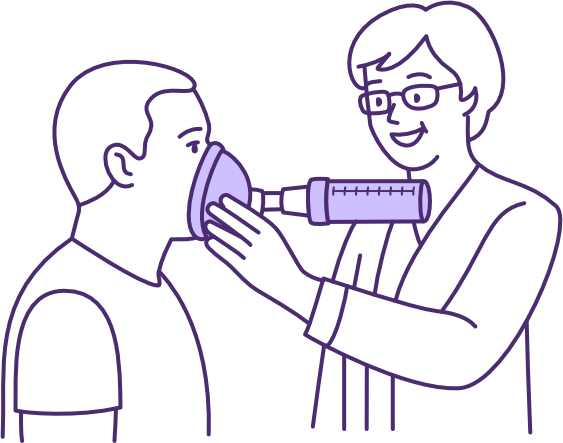
Use of a LVR bag for air stacking to improve lung volume, measured by improved peak cough flow following SCI
Image source QSCIS
The lung volume recruitment (LVR) bag facilitates lung volume augmentation using a technique known as “lung volume recruitment (LVR)” or “air stacking”. Multiple insufflations are manually delivered in addition to the user’s own inspiratory effort, until maximal insufflation capacity (MIC) is reached, before disconnecting the user to permit exhalation.
LVR requires the user to have full glottal function, as well as be able to breathe spontaneously and cooperate with the technique, preferably using a mouthpiece and nose clip. The LVR bag is a low-cost, non-powered, and highly portable device, with application for the appropriate user in both hospital and community settings.
It is important to note that, while the LVR bag may have a general pressure gauge, the LVR bag mechanism does not provide any control over the tidal volume delivered and the inspiratory pressure generated. Rather the technique is dependent on the user’s cooperation to indicate when their MIC is reached and provide a clear communication signal to the operator to cease insufflations.
Hence, there is also no capacity to pre-program LVR settings to standardise lung volume augmentation, but it can still be delivered across a 24-hour period by trained non-physiotherapy staff, including nurses, carers, and family members. However, training is essential to ensure safe and effective delivery, especially to minimise the risk of volutrauma/barotrauma.
If appropriate, LVR can provide the benefits of lung volume augmentation, while reducing the inspiratory effort required. However, repeated coughing efforts following LVR may contribute to respiratory fatigue over time. For these reasons, mechanical insufflation–exsufflation (MI-E) devices are recommended when available for lung volume augmentation, to assist the standardisation of therapy and reduce the work of breathing associated with treatment.
LVR kit explanation
A LVR kit consists of a manual resuscitator bag with a general pressure gauge, modified with a one-way valve, a flexible tubing piece, via a mouthpiece and nose clip. A bacterial filter may also be included in the circuit to support airway hygiene. The LVR bag does not deliver aerosolised medications and typically operates using room air only.
The one-way valve alters the function of the resuscitator bag by allowing airflow only in the direction of insufflation and preventing leakage between stacked insufflations. As a result, the LVR bag is no longer suitable for resuscitation, and exhalation into the circuit is contraindicated due to the risk of barotrauma. Instead, the interface must be detached from the user once the final insufflation volume is reached, prior to their exhalation.
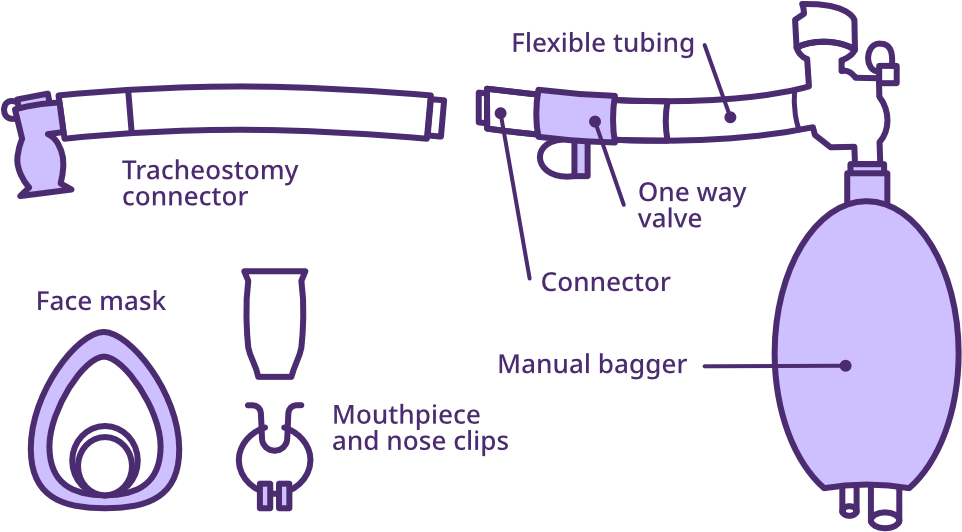
Lung volume recruitment (LVR) bag kit
Adapted from SCIRE Professional
LVR method
- The user first takes a deep breath, according to their maximal inspiratory capacity.
- The user then closes their glottis to hold their inspiratory volume, while being connected to the LVR bag.
- The user then reopens their glottis, to allow the LVR bag to deliver additional air volume—this is stacked onto their own maximal inspiratory capacity by a series of squeezes, until the user’s MIC/comfort limit is reached and they signal to cease insufflation (typically up to five small squeezes of the LVR bag).
- The user then closes their glottis to hold the stacked inspiratory capacity, while being disconnected from the LVR bag.
- The user then opens their glottis to exhale, cough (+/- manual assisted cough), huff, speak or sing, as desired.
LVR variations
Additional features include:
- tracheostomy connector, provided the tracheostomy has a deflated cuff or is cuffless to permit glottal control, but these must be used with extreme caution due to the risk of volutrauma/barotrauma
- use of a mouthpiece with nose clip is highly preferred to a non-vented mask, so that the user can control detachment from the LVR bag as necessary
- some users may be able to self-administer the technique—for example, by positioning the bag between the elbow and trunk and squeezing it like a bagpipe; this offers additional safety, given the user is able to control insufflation within their comfort range; however, they must also be able to disconnect from the device for each exhalation.
LVR clinical reasoning

Graph of spirometry indicators for insufflation and assisted coughing, including LVR
Toussaint, M., Chatwin, M., González, J., Berlowitz, D. J., & ENMC Respiratory Therapy Consortium. (2018). 228th ENMC International Workshop: Airway clearance techniques in neuromuscular disorders, Naarden, The Netherlands, 3–5 March, 2017. Neuromuscular Disorders, 28(3), 289–298. https://dhttps://doi.org/10.https://www.nmd-journal.com/article/S0960-8966(17)30588-6/fulltext#f00101016/j.nmd.2017.10.008
LVR more information
More information about LVR can be found here.
Exhalation into the LVR bag is contraindicated, due to the presence of a one-way valve and the risk of volutrauma/barotrauma.
Prior to exhalation, the LVR bag must be detached from the user.
LVR therapy cannot be used with endotracheal intubation, as glottal function is bypassed.
If used with a tracheostomy, it must be cuffless/cuff deflated prior to therapy sessions, to permit glottal function.
Hyperinflation
The Australian and New Zealand Physiotherapy Guidelines for people with SCI states:
Consensus: Strong for recommendation
Intermittent application of positive pressure therapy techniques should be used (in consultation with medical staff) for improving lung volume in ventilated people with acute SCI that are medically stable.
Positive pressure therapy techniques include ventilator hyper-inflation and manual-hyperinflation.
Ventilator hyperinflation is preferred if available.
Manual hyperinflations, also known as “bagging,” is a technique used for lung volume augmentation in the context of invasive mechanical ventilation. It involves the use of a manual resuscitation bag connected to an endotracheal or tracheostomy tube, typically with a bacterial filter added for airway hygiene.
Unlike an LVR bag, this setup does not include a one-way valve, allowing the circuit to remain connected during passive exhalation. The bag can also be used for resuscitation if required.
The technique involves manually delivering insufflations, usually with staged increases in inspiratory volume. Supplemental oxygen may be included as clinically indicated. However, manual hyperinflations are more difficult to standardise, with an increased risk of volutrauma/barotrauma and tension pneumothorax.
For this reason, it should be performed only by physiotherapists or trained intensive care staff.
Where available, ventilator delivered hyperinflation is recommended as a safer and more consistent alternative.
Deep breathing exercises
The Australian and New Zealand Physiotherapy Guidelines for people with SCI states:
Consensus: Strong for recommendation
Deep breathing exercises may be provided to improve lung volumes in people with SCI.
People with SCI and respiratory muscle weakness should focus on respiratory strength training exercises, rather than deep breathing exercises.
Historically, physiotherapy deep breathing exercises can range from the use of incentive spirometry, active cycles of breathing techniques (ACBT) to demand ventilation activities. Following SCI, these exercises may be appropriate to use for lung volume augmentation and other benefits at some stage, but have risks and limitations—particularly in the acute phase of management.
Deep breathing exercises rely on voluntary respiratory muscle strength to access inspiratory reserve volumes. Overall, due to respiratory muscle weakness, there is likely to be a ceiling effect on the maximal lung volumes achieved with voluntary deep breathing effort. This ultimately limits the efficacy of this intervention, in comparison to other types of lung volume augmentation techniques.
When acutely unwell, deep breathing exercises may also accelerate the onset of respiratory fatigue due to altered breathing mechanics and airway resistance increasing the work of breathing. Hence, they are not appropriate at this time.
Once a person with SCI is medically stable, deep breathing exercises may be introduced using a graduated approach, but typically would be better incorporated following:
- positive-pressure breathing techniques to improve lung and chest wall compliance
- respiratory muscle training to improve voluntary inspiratory muscle strength and capacity.
Inspiratory muscle training (IMT)
The Australian and New Zealand Physiotherapy Guidelines for people with SCI states:
Evidence: Weak for recommendation
Respiratory muscle training may be used to improve respiratory muscle strength in people with SCI who have respiratory muscle weakness.
Respiratory muscle training most commonly involves inspiratory muscle training but can also include expiratory muscle training
Expiratory muscle training (EMT) aims to improve diaphragm positioning, increase expiratory flow rates and volumes and enhance postural stability while maintaining ventilation.
Inspiratory muscle training (IMT) aims to improve the strength of weakened (not paralysed) inspiratory muscles to:
- improve diaphragm function, by improving diaphragm contraction and chest wall stability during inspiration
- increase tidal and inspiratory reserve volumes, for improved minute ventilation and deep breathing benefits
- augment expiratory volumes, via enlarged inspiratory volumes
- enhance postural stability, via improved diaphragm activity and transdiaphragmatic pressure generation.
To guide the development of an IMT program, it is useful to:
- identify the acute or chronic respiratory goals and benefits: IMT may
- facilitate readiness for weaning and decannulation by
- improving voluntary tidal and inspiratory reserve volumes for improved minute ventilation and addressing the risks associated with hypoventilation
- reducing the risk of respiratory fatigue related to increased work of breathing from
- poor diaphragm positioning and contraction strength during inspiration
- poor accessory muscle contraction strength during inspiration
- inadequate inspiratory reserve volumes for deep breathing and coughing
- enhance dynamic postural control, including introduction of sitting or standing, while still maintaining ventilation
- build inspiratory capacity and reserve for
- speech volume and phrase length
- engagement in rehabilitation
- activities of daily living etc.
- chronic periods of ill health or confinement to bed
- slow respiratory function decline which is associated with chronic SCI and ageing.
- facilitate readiness for weaning and decannulation by
- identify the key respiratory muscles and types of exercises/activities: IMT could target
- strengthening the diaphragm, external intercostal and inspiratory accessory muscles
- for improved diaphragm efficiency and lung volume generation
- to improve recruitment of a weakened hemi-diaphragm
- for core stability tasks, while maintaining ventilation
- complementary stretching of the trunk and associated chest wall, including use of positive-pressure breathing devices to also improve lung and chest wall compliance
- complementary stretching of the abdominal muscles—if hypertonicity is present—to reduce inspiratory resistance to the descent of the diaphragm
- strengthening the diaphragm, external intercostal and inspiratory accessory muscles
- identify the type of training effects: IMT could incorporate
- resistance training via high intensity contractions with few repetitions
- endurance training via low-intensity contractions, repeated or held over an extended period of time
- specific exercises versus activity specific task and training approaches
- complementary stretching
- identify useful baseline measures: IMT could utilise
- spirometry, such as maximal inspiratory pressure (MIP) and maximal sniff nasal inspiratory pressure (SNIP), but also expiratory volume and flow rate measures which indirectly measure inspiratory capacity
- other measures e.g. ventilator free breathing period over 24 hours, Borg RPE scale, speech and balance measures.
IMT method
Using resistance principles similar to conventional muscle training, one simple method involves placing an appropriate weight on the abdomen in supine, creating resistance to abdominal rise—as the diaphragm descends during inspiration. Most commonly, IMT is typically delivered through an electronic threshold device via a mouthpiece, which can be set to incrementally increase the effort required for inspiration as inspiratory muscle strength improves.
Training can be performed in various positions, including supine or upright sitting—wearing an abdominal binder to optimise diaphragm positioning. Palpation of both abdominal rise and chest wall movement can be used with verbal coaching, to maximise effort.
Training recommendations suggest completing IMT sessions up to twice daily, five days per week, for 6–8 weeks or longer. Resistance should be progressively increased, with intensity potentially guided by the Borg RPE scale.
Similar to conventional exercise programs, strength gains are maintained only while the exercise program continues.
IMT example program
An IMT program can be found here.
IMT video information
Some IMT instruction videos can be found here.
Discharge and community planning
A person with SCI who has ongoing and significant respiratory dysfunction, will require ongoing ventilation and respiratory health supports for community living. Hospital discharge planning processes will need to address funding, care recruitment and training, as well as establish an adequate network of health professional support. A comprehensive process of trialling and scripting will also be important to supply necessary respiratory equipment and consumables.
For further information, refer to Discharge and community planning.
Physiotherapy management of people with spinal cord injury (2022)
The Australian and New Zealand Physiotherapy Guidelines for people with SCI
Respiratory education modules and YouTube channel
Canadian Alternatives in Noninvasive Ventilation (CANVent)
Girdle/Abdominal binder
SCIRE Professional
Cough techniques for individuals with tracheostomy
Tracheostomy Education
IPPB via the Servo I Guidelines for use in UCH Critical Care
University College London Hospitals
Mechanical insufflation-exsufflation (MI-E)
Canadian Alternatives in Noninvasive Ventilation (CANVent)
Lung volume recruitment with manual resuscitation bag (LVR bag)
Canadian Alternatives in Noninvasive Ventilation (CANVent)
Cough assist and secretion removal
SCIRE Professional
Manual hyperinflation
Physiopedia
Active cycle of breathing technique
Physiopedia
Inspiratory muscle retraining in people with spinal cord injury
Agency for Clinical Innovation (ACI) NSW
Respiratory muscle training
SCIRE Professional
Inspiratory muscle training
SCIRE Community
Brown, R., DiMarco, A. F., Hoit, J. D., & Garshick, E. (2006). Respiratory dysfunction and management in spinal cord injury. Respiratory Care, 51(8), 853-870. https://doi.org/10.4187/respircare.05185
Berlowitz, D. J., Wadsworth, B., & Ross, J. (2016). Respiratory problems and management in people with spinal cord injury. Breathe (Sheff), 12(4), 328–340. https://doi.org/10.1183/20734735.012616
Bissett, B., Gosselink, R., & van Haren, F. M. P. (2020). Respiratory muscle rehabilitation in patients with prolonged mechanical ventilation: A targeted approach. Critical Care, 24(1), 103. https://doi.org/10.1186/s13054-020-2783-0
Chatwin, M., Toussaint, M., Gonçalves, M. R., Sheers, N., Mellies, U., Gonzales-Bermejo, J., Sancho, J., Fauroux, B., Andersen, T., Hov, B., Nygren-Bonnier, M., Lacombe, M., Pernet, K., Kampelmacher, M., Devaux, C., Kinnett, K., Sheehan, D., Rao, F., Villanova, M., Berlowitz, D. J., & Morrow, B. M. (2018). Airway clearance techniques in neuromuscular disorders: A state of the art review. Respiratory Medicine, 136, 98–110. https://doi.org/10.1016/j.rmed.2018.01.012
Chatwin, M., & Wakeman, R. H. (2023). Mechanical insufflation-exsufflation: Considerations for improving clinical practice. Journal of Clinical Medicine, 12(7), 2626. https://doi.org/10.3390/jcm12072626
Denehy, L., & Berney, S. (2001). The use of positive pressure devices by physiotherapists. European Respiratory Journal, 17(4), 821–829. https://doi.org/10.1183/09031936.01.17408210
Denton, M., & McKinlay, J. (2009). Cervical cord injury and critical care. Continuing Education in Anaesthesia, Critical Care & Pain, 9(3), 82–86. https://doi.org/10.1093/bjaceaccp/mkp013
Galeiras Vázquez, R., Rascado Sedes, P., Mourelo Fariña, M., Montoto Marqués, A., & Ferreiro Velasco, M. E. (2013). Respiratory management in the patient with spinal cord injury. BioMed Research International, 2013, Article 168757. https://doi.org/10.1155/2013/168757
Harvey, L. A. (2008). Management of spinal cord injuries: A guide for physiotherapists. Churchill Livingstone Elsevier.
Jeong, J. H., & Yoo, W. G. (2015). Effects of air stacking on pulmonary function and peak cough flow in patients with cervical spinal cord injury. Journal of Physical Therapy Science, 27(6), 1951–1952. https://doi.org/10.1589/jpts.27.1951
Mueller, G., de Groot, S., van der Woude, L., & Hopman, M. T. E. (2008). Time-courses of lung function and respiratory muscle pressure generating capacity after spinal cord injury: A prospective cohort study. Journal of Rehabilitation Medicine, 40(4), 269–276. https://doi.org/10.2340/16501977-0172
Randelman, M., Zholudeva, L. V., Vinit, S., & Lane, M. A. (2021). Respiratory training and plasticity after cervical spinal cord injury. Frontiers in Cellular Neuroscience, 15, Article 700821. https://doi.org/10.3389/fncel.2021.700821
Reyes, M. R. L., Shaffer, J., Kwong, C., & Gater, D. (2020). A primary care provider’s guide to managing respiratory complications after spinal cord injury. Top Spinal Cord Inj Rehabil, 26(2), 116–124. https://doi.org/10.46292/sci2602-116
Toussaint, M., Chatwin, M., González, J., Berlowitz, D. J., & ENMC Respiratory Therapy Consortium. (2018). 228th ENMC International Workshop: Airway clearance techniques in neuromuscular disorders, Naarden, The Netherlands, 3–5 March, 2017. Neuromuscular Disorders, 28(3), 289–298. https://doi.org/10.1016/j.nmd.2017.10.008
Woods, A., Gustafson, O., Williams, M., & Stiger, R. (2022). The effects of inspiratory muscle training on inspiratory muscle strength, lung function and quality of life in adults with spinal cord injuries: A systematic review and meta-analysis. Disability and Rehabilitation, 45(4), 1-12. https://doi.org/10.1080/09638288.2022.2107085