Normal respiratory function
The work of breathing
Under normal conditions, the work of breathing is minimal at rest and during low-intensity activity.
As metabolic demand increases, the work of breathing also increases to maintain adequate ventilation.
With extreme exertion, the work of breathing becomes unsustainable over time, eventually leading to fatigue or exhaustion—depending on individual cardiorespiratory fitness.
Central and autonomic nervous system roles
Respiration depends on the coordinated function of the central and autonomic nervous systems.
The central nervous system (CNS)—primarily the brainstem, including the medulla oblongata and pons—regulates the rhythmic pattern of breathing by generating and modulating respiratory drive. These brain centres integrate various inputs to adjust the rate and depth of breathing in response to changing metabolic demands.
The CNS—primarily the brain to spinal segment pathways—regulates the strength, tone and associated compliance of respiratory muscles. It is also the central pattern generator for respiratory motor function. Distinct neural patterns of muscle recruitment govern inspiration and passive expiration, as well as forced expiratory actions such as sighing, coughing and sneezing.
The autonomic nervous system (ANS)—particularly its sympathetic and parasympathetic branches—modulates pulmonary blood flow, airway reactivity, sputum production and further changes to respiratory rate, so that:
- sympathetic activation promotes bronchodilation and increases in respiratory rate
- parasympathetic input contributes to bronchoconstriction and associated sputum production.
Diaphragm roles
The diaphragm, is a large, domed muscle located beneath the lung bases. It separates the thoracic and abdominal cavities, and is the primary muscle recruited for respiratory actions.
The diaphragm generates dynamic transdiaphragmatic pressures through its selective contraction and relaxation. This means:
- during inspiration, the diaphragm contracts and descends, creating negative intrathoracic pressure and positive intra-abdominal pressure which generates at least 65% of inspiratory volume
- during passive expiration, the diaphragm relaxes, reversing these pressures
- during forced expiration, the diaphragm co-contracts with abdominal, pelvic floor, internal intercostal, and intrinsic laryngeal muscles to regulate intra-abdominal, intrathoracic, and subglottic pressures. This controls airflow for forced expiratory actions e.g. coughing, as well as more complex functions e.g. singing.
The diaphragm’s positioning (between the thoracic and abdominal cavities) and dome-shape, along with its capacity to generate varying transdiaphragmatic pressures, also:
- aids venous and lymphatic return
- supports lower oesophageal sphincter function to limit reflux
- contributes to dynamic postural stability.
Inspiration
Normal diaphragmatic contraction not only requires intact spinal nerve root innervation, but it also requires normal intercostal and abdominal muscle tone and compliance.
Normal intercostal muscle tone stabilises the chest wall and intercostal spaces against increasing negative intrathoracic pressures as the diaphragm contracts and descends. This prevents the collapse of the chest wall and development of intercostal recessions during inspiration. A stabilised chest wall also provides a stable base for the diaphragm to contract against during later inspiration, enhancing inspiratory volume further.
Normal abdominal muscle tone positions the abdominal contents against the diaphragm, creating its dome-shape at rest, while supporting its shape during contraction. This shaping is critical to permit the diaphragm to contract through a greater excursion range, from fully domed to flattened. By optimising the diaphragm’s length-tension relationship throughout inspiration, inspiratory volumes are generated more efficiently.
At the same time, normal intercostal and abdominal muscle compliance permits the expansion of the chest wall during inspiration. Similarly, it allows the abdominal contents to be displaced by the diaphragm’s descent, causing some protrusion of the abdominal wall during inspiration—especially in sitting. This normal compliance permits the diaphragm to complete its full excursion and generate maximal inspiratory volume when required.
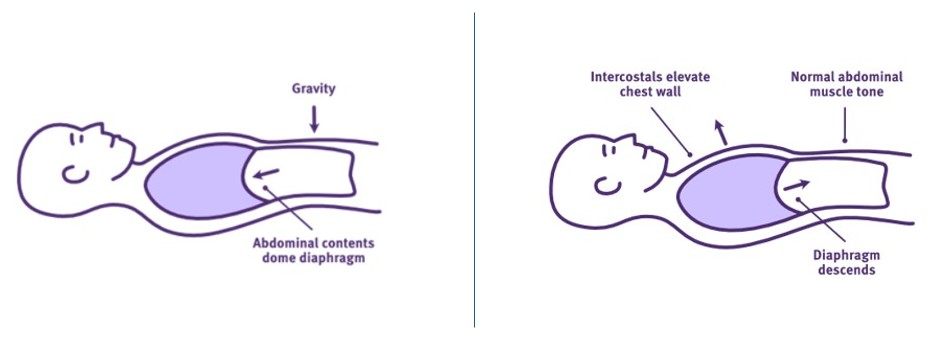
Intercostal and abdominal muscle tone and compliance impacts diaphragm function
Adapted from Thoracic Key
Active contraction of the scalene and external intercostal muscles assists rib cage elevation, along with the final stage of diaphragm contraction to expand the chest wall further and increase inspiratory lung volume. Other accessory muscles such as sternocleidomastoid may also be recruited to increase inspiratory force, flow rates or the overall respiratory rate as needed.
Expiration
Expiration is primarily a passive process that occurs when the diaphragm relaxes and recoils back to its original dome-shape. In turn, the negative intrathoracic pressure reduces, allowing the elastic recoil of the lungs and chest wall too.
This recoil assists with regulating CO₂ levels, enhanced by periodic sighing—a gentle, forced expiration. However, secretion clearance typically requires more powerful, forced expiration via coughing, huffing, sneezing and/or nose-blowing. Other accessory muscles may be recruited to further increase expiratory volumes, force and flow rates as needed.
Normal breathing pattern
The normal breathing pattern is:
- abdominal rise during inspiration – due to the descent and flattening of the diaphragm into the abdomen, with normal abdominal wall compliance
- lateral expansion of the lower ribs – due to activity of the external intercostals, but also the final stages of diaphragm contraction from its insertion on the lower ribs
- anterior-posterior elevation of the rib cage – due to the activity of the external intercostals and other accessory muscles
- abdominal fall during expiration – due to the relaxation of the diaphragm, allowing the abdominal contents to track against the relaxing diaphragm and re-establish its dome-shape at rest.
The following video (up to 1min 44sec) illustrates the normal breathing pattern generated by the synergistic action of the diaphragm, intercostal and abdominal muscles.
Normal ventilation
Volume and flow rate
To achieve optimal gas exchange, ventilation ultimately depends on the degree of air volume and flow rate, entering and exiting the lungs.
Various aspects of lung volume and flow rate can be measured using spirometry, for comparison with normalised values determined by sex, age, height, weight and ethnicity. Measures of respiratory muscle strength can also be completed, which indicate capacity to generate lung volumes and flow rates.
Measures of respiratory function
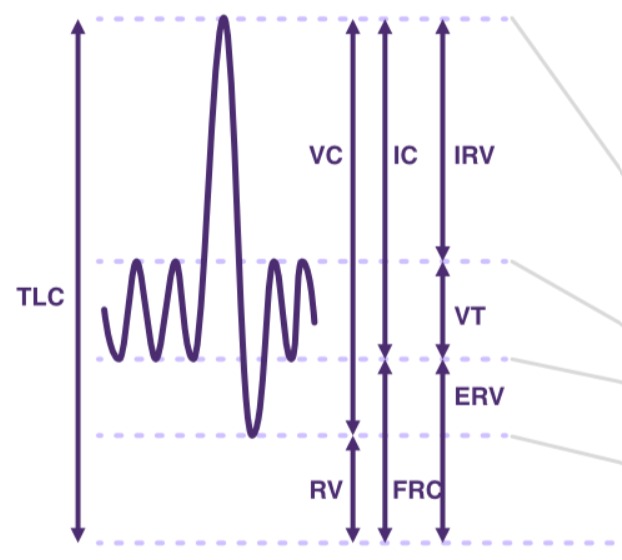
Measures of lung volumes and capacities
Adapted from SCIRE Professional
Lung volumes
Lung volumes are measured in mL or L.
- Tidal Volume (TV or VT) is the volume inhaled or exhaled per breath.
- Minute Ventilation (VE) is an important indicator of overall ventilation and is calculated as follows: VE= average VT x respiratory rate (RR) in L/min.
- Inspiratory Reserve Volume (IRV) and Expiratory Reserve Volume (ERV) are the additional volumes beyond this tidal volume that can be inhaled or exhaled.
- Forced Expiratory Volume (FEV1) is the volume exhaled in the first second of a forced exhalation, after a full inhalation.
- Residual Volume (RV) is the volume remaining in the lungs after maximal exhalation (can only be measured directly using plethysmography).
Lung capacities
Lung capacities are combinations of volumes and are measured in mL or L.
- Total Lung Capacity (TLC) = VT + IRV + ERV + RV reflects maximum lung volume.
- Vital Capacity (VC) = VT + IRV + ERV is the total volume maximally and slowly exhaled, after a full inhalation.
- Forced Vital Capacity (FVC) may = VT + IRV + ERV (unless affected by obstructive airway disease) is the total volume maximally and quickly exhaled, after a full inhalation.
- Functional Residual Capacity (FRC) = ERV + RV is the air remaining in the lungs after normal exhalation (can only be measured directly using plethysmography).
Lung flow rates
Lung flow rates are measured in L/min.
- Peak Expiratory Flow (PEF) is the maximal flow rate that can be exhaled when breathing out as fast as possible (which measures maximum expiratory flow, after a full inspiration, through an open glottis), after a full inhalation.
- Peak Cough Flow (PCFunassisted) is the maximal flow that can be exhaled when coughing as strong as possible (during the compressive phase of a cough, just after sudden opening of the glottis), after a full inhalation.
- Peak Cough Flow (PCFassisted) is the maximal flow rate that can be exhaled via coughing as strong as possible— timed with the application of a manual assisted cough (during the compressive phase of a cough, just after sudden opening of the glottis); it can also be compared with the maximal flow rate that can be exhaled using a lung volume augmented breath prior to the application of a manual assisted cough.
Normal predictive ranges
Normal predictive volumes, capacities and peak flow values for adults are dependent on sex, age, height, weight and ethnicity. Variations in testing procedure and patient effort also impact results.
Normal adult VT is typically 6–8 mL/kg.
Normal adult FVC ranges from 2.5-5.5L or 60-70mL/kg.
Normal adult TLC is approximately 4-6L.
Normal adult FEV1 accounts for approximately 75-85% of the FVC.
Normal adult Functional Expiratory Ratio (FER = FEV1/FVC) is approximately 0.7.
Normal adult PCF ranges from 360-840 L/min.
Measures of respiratory muscle strength
Respiratory muscle strength
Respiratory muscle strength is measured in cmH20.
- Maximal Inspiratory Pressure (MIP) is a measure of the strength of the diaphragm and other accessory muscles, as well as lung and chest wall compliance, performed by actively exhaling fully to residual volume before measuring a maximal inhalation over 3 seconds via a mouthpiece (-value cmH20).
- Sniff Nasal Inspiratory Pressure (SNIP) is a measure of the strength of the diaphragm, along with oesophageal tone, performed by actively exhaling fully to residual volume before measuring a maximal inhalation over 3 seconds via 1 nostril (+value cmH20).
- Maximal Expiratory Pressure (MEP) is a measure of the strength of abdominal and other expiratory muscles, performed by actively inhaling to total lung capacity before measuring a maximal exhalation over 3 seconds via a mouthpiece (+value cmH20).
Normal predictive ranges
Normal predictive respiratory muscle strength values for adults are particularly dependent on sex and age, but also weight, height or body mass index (BMI). Variations in testing procedure and patient effort also impact results.
Algorithms can be used to calculate predicted values for sex and age, including the lower limits of normal (LLN). The following is an example:
Normal male MIP = 120 − (0.41 × age), and male MIP LLN = 62 − (0.15 × age)
Normal male MEP = 174 − (0.83 × age), male MEP LLN = 117 − (0.83 × age)
Normal female MIP = 108 − (0.61 × age), and female MIP LLN = 62 − (0.50 × age)
Normal female MEP = 131 − (0.86 × age), and female MEP LLN = 95 − (0.57 × age).
Evans, J. A., & Whitelaw, W. A. (2009). The assessment of maximal respiratory mouth pressures in adults. Respiratory Care, 54(10), 1348–1359. https://doi.org/10.4187/respcare.09541348
Airway resistance
As discussed above, respiratory muscle recruitment produces the mechanical action of inspiration, followed by passive expiration. Effectively, they generate the pressure gradient needed to overcome innate airway resistance and drive a specific volume and flow rate of air in and out of the lungs.
Airway resistance is determined by a range of factors such as airway diameter. Smaller airways, like bronchioles, naturally have higher resistance compared to larger airways, like the trachea. As lung volume decreases during expiration, airways narrow and increase resistance.
A combination of any, or all of the following, can further reduce the compliance of the lungs and chest wall, increasing airway resistance:
- bronchospasm (increased airway smooth muscle tone)
- atelectasis (alveoli collapse)
- secretions (sputum etc.)
- pulmonary oedema (swelling internal or external to the alveoli)
- pleural effusions (swelling in the pleural space)
- rigidity of the chest wall (rib cage and muscles).
Gas exchange
Ventilation is the gaseous exchange of O₂ and CO₂ which occurs via the lungs.
The degree of gas exchange can be measured using various methods, including the analysis of blood or exhaled air.
Measures of gas exchange
Pulse oximetry
Along with a measure of heart rate, pulse oximeters provide a non-invasive measure of the oxygen saturation level in the blood, determined by the degree of probe light absorbed by oxygenated and deoxygenated haemoglobin.
- Peripheral oxygen saturation (SpO2) indicates the percentage of haemoglobin in the peripheral circulating blood that is saturated with oxygen. A normal range is 95-100%.
Blood gas analysis
Blood gas analysis can measure the following indicators of ventilation.
- Partial pressure of oxygen (PaO2) indicates the amount of oxygen dissolved in the blood, reflecting how well oxygen is moving from the lungs into the bloodstream. A normal range is typically 80-100 mmHg.
- Partial pressure of carbon dioxide (PaCO2) indicates the amount of carbon dioxide in the blood, indicating how well carbon dioxide is being removed from the body. A normal range is typically 35-45 mmHg.
- pH indicates the acidity or alkalinity of the blood. The body’s regulation of blood pH determines the overall acid-base balance, which must be tightly controlled to maintain normal function of many organs. One key mechanism involves the respiratory system: cells constantly produce CO₂ as a metabolic byproduct, which lowers blood pH as it accumulates. The brain regulates breathing rate and depth to adjust CO₂ exhalation—faster, deeper breathing removes more CO₂, raising pH. This allows the respiratory system to adjust blood pH on a minute-to-minute basis. A normal pH range is typically 7.35-7.45.
- Bicarbonate (HCO3) indicates the amount of the basic compound bicarbonate in the blood, which helps maintain acid-base balance. A normal range is typically 22-26mEq/L.
Exhaled air analysis
- There are a range of non-invasive techniques which examine the chemical composition of an exhaled air sample e.g capnography which measures the maximum CO2 concentration at the end of expiration and provides real time analysis of ventilation, perfusion and metabolism.
Overall, any adverse and sustained changes to these aspects of ventilation- lung volumes and flow rates, airway resistance and gas exchange- will increase the work of breathing to meet the physiological needs of respiration.
Normal airway clearance
The internal epithelial layer of the whole tracheobronchial tree produces sputum, which is a thick, viscous secretion. It consists of mucus, which mops up inflammatory cells and traps inhaled cellular particles, pathogens, and debris.
The mucociliary escalator is comprised of hair-like cilia which extrude from the epithelium and beat in a coordinated manner. This sweeps sputum, aided by expiratory airflow, toward the oropharynx for clearance via swallowing or expectoration (coughing).
Coughing—either reflexive or voluntary—is the body’s primary “host defence” mechanism for clearing the airway of secretions and debris, as well as inhaled and aspirated foreign matter. An effective cough consists of three phases, which all require unique transdiaphragmatic pressures:
- inspiration – maximal inspiratory muscle contraction of the diaphragm and accessory muscles to achieve a full volume of inhaled air within the lungs
- compression – glottic closure to temporarily close the airway
- expulsion – forceful expiratory muscle contraction of the abdominal and internal intercostal muscles, combined with rapid glottic opening to suddenly open the airway; together this allows the sudden and forced exhalation of the full volume of air from the lungs.
Normal respiratory muscle strength, including the inspiratory volume prior to a cough, ultimately determines the cough expiratory volume and flow rate. In a healthy adult, producing an effective cough is estimated to require at least 50%—but typically 80-90%—of inspiratory lung volume and should generate a peak cough flow >360 L/min.
For more information, refer to Respiratory muscles.
How the lungs work—the respiratory system
National Heart, Lung and Blood Institute
Berry, R. B. (2012). Sleep and respiratory physiology. In Fundamentals of Sleep Medicine (pp. 141–158). Elsevier/Saunders. ISBN 978-1-4377-0326-9
Brennan, M., McDonnell, M. J., Duignan, N., Gargoum, F., & Rutherford, R. M. (2022). The use of cough peak flow in the assessment of respiratory function in clinical practice: A narrative literature review. Respiratory Medicine, 193, 106740. https://doi.org/10.1016/j.rmed.2022.106740
Chen, R., & Guyenet, P. G. (Eds.). (2022). Respiratory Neurobiology: Physiology and Clinical Disorders, Part II (Vol. 189). In M. J. Aminoff, F. Boller, & D. F. Swaab (Series Eds.), Handbook of Clinical Neurology. Elsevier. ISBN 978-0-323-91532-8
DiMarco, A. F., Kowalski, K. E., & Supinski, G. (2005). Restoration of respiratory muscle function following spinal cord injury: Review of electrical and magnetic stimulation techniques. Respiratory Physiology & Neurobiology, 147(2–3), 273–287. https://doi.org/10.1016/j.resp.2005.07.012
Donley, E. R., Holme, M. R., & Loyd, J. W. (2024). Anatomy, thorax, wall movements. In StatPearls. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK526023/
Evans, J. A., & Whitelaw, W. A. (2009). The assessment of maximal respiratory mouth pressures in adults. Respiratory Care, 54(10), 1348–1359. https://doi.org/10.4187/respcare.09541348
Fogarty, M. J., Mantilla, C. B., & Sieck, G. C. (2018). Breathing: Motor control of diaphragm muscle. Physiology (Bethesda), 33(2), 113–126. https://doi.org/10.1152/physiol.00002.2018
Gil Obando, L. M., López López, A., & Ávila, C. L. (2012). Normal values of the maximal respiratory pressures in healthy people older than 20 years old in the City of Manizales – Colombia. Colombia Médica (Cali), 43(2), 119-125. https://doi.org/10.25100/cm.v43i2.2489
Hodges, P. W., Butler, J. E., McKenzie, D. K., & Gandevia, S. C. (1997). Contraction of the human diaphragm during rapid postural adjustments. The Journal of Physiology, 505(Pt 2), 539–548. https://doi.org/10.1111/j.1469-7793.1997.539bb.x
Sclauser Pessoa, I. M., Franco Parreira, V., Fregonezi, G. A., Sheel, A. W., Chung, F., & Reid, W. D. (2014). Reference values for maximal inspiratory pressure: A systematic review. Canadian Respiratory Journal, 21(1), 43–50. https://doi.org/10.1155/2014/982374
Smith, J. A., Aliverti, A., Quaranta, M., McGuinness, K., Kelsall, A., Earis, J., & Calverley, P. M. (2012). Chest wall dynamics during voluntary and induced cough in healthy volunteers. The Journal of Physiology, 590(3), 563–574. https://doi.org/10.1113/jphysiol.2011.213157
U.S. National Library of Medicine. (2021). Cough and airway clearance strategies in SCI (NBK541029). In NCBI Bookshelf. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK541029/
U.S. National Library of Medicine. (2021). Pulmonary function test: Spirometry (NBK560526). In NCBI Bookshelf. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK560526/
U.S. National Library of Medicine. (2021). Respiratory assessment after spinal cord injury (NBK560880). In NCBI Bookshelf. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK560880/
U.S. National Library of Medicine. (2021). Spirometry in neuromuscular disease (NBK540970). In NCBI Bookshelf. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK540970/
Watson, A. (2014). Breathing in singing. In The Oxford handbook of singing (Chapter 10). https://doi.org/10.1093/oxfordhb/9780199660773.013.10
Willis, L. D. (2023). Cough peak flow assessment: Is there more to the story? Respiratory Care, 68(4), 553–555. https://doi.org/10.4187/respcare.10900
Yu, X., Jiang, H.-y., Zhang, C.-x., Jin, Z.-h., Gao, L., Wang, R.-d., Fang, J.-p., Su, Y., Xi, J.-n., & Fang, B.-y. (2021). The role of the diaphragm in postural stability and visceral function in Parkinson’s disease. Frontiers in Aging Neuroscience, 13, Article 785020. https://doi.org/10.3389/fnagi.2021.785020
Zimmer, M. B., Nantwi, K., & Goshgarian, H. G. (2007). Effect of spinal cord injury on the respiratory system: Basic research and current clinical treatment options. The Journal of Spinal Cord Medicine, 30(4), 319–330. https://doi.org/10.1080/10790268.2007.11753947