Respiratory assessment
A comprehensive respiratory assessment is essential to complete, both in the context of an acute spinal cord injury (SCI) and for a person with chronic SCI, who becomes acutely unwell.
The goals of a respiratory function assessment are to:
• establish a baseline respiratory status
• identify changes in respiratory function and associated predictive factors for complications
• inform acute management planning and guide timely interventions
• support monitoring for potential deterioration, particularly during acute management.
Performing a respiratory assessment
The following are some key aspects of assessing respiratory function in a person with SCI.
Positioning
Positioning
The optimal position for assessing acute respiratory function is supine, as this position reduces the work of breathing and allows access for a range of assessments and interventions.
Ideally, head elevation should remain less than 30 degrees to minimise the risk of sacral skin shearing, while cases involving abdominal distension, obesity, or chronic spinal cord injury, a modified supine position with slight head elevation may be required.
Consider whether any of the following may affect capacity to be managed in supine:
- spinal alignment or other medical/orthopaedic precautions
- risk of aspiration in supine
- areas of skin breakdown.
General observations
General observations
Complete general observations of key clinical signs and note:
- blood gases and respiratory rate, within the context of any supplemental oxygen and/or ventilation supports
- continuous pulse oximetry for at least 48-72 hours and beyond, until signs of spinal shock resolve and respiratory function stabilises
- regular arterial blood gas and capnography monitoring, as pulse oximetry will not be sufficient to detect early signs of respiratory deterioration
- hypotension, bradycardia, or hypothermia, which is often secondary to ANS dysfunction
- pyrexia/fever, which may indicate respiratory complications such as pulmonary embolism or pneumonia
- pain, including capacity to manage normal tidal breathing versus higher inspiratory volumes and cough
- consciousness, particularly capacity to manage sputum and saliva clearance, follow instructions and communicate.
Auscultation
Auscultation
Auscultate all lung fields, with particular attention to the bases and note:
- the degree and symmetry of air entry
- the presence of any added sounds (e.g. crackles or wheezes), especially if aspiration is suspected
- the presence of additional tactile fremitus and external respiratory sounds.
Lung volume augmentation with a positive pressure breathing device may assist auscultation further.
Spirometry
Spirometry
Spirometry, also known as respiratory function tests (RFTs) provide objective measures of respiratory muscle strength, lung capacity and forced expiratory flow rates.
Spirometry is valuable to:
- monitor respiratory function deterioration or improvement
- guide use of ventilation support, including weaning and decannulation
- evaluate the effectiveness of interventions, including lung volume augmentation and secretion management
- educate on strategies such as abdominal binder use in sitting to support diaphragm function.
Following SCI, changes from predicted spirometry values typically correlate with the neurological level of injury (NLI) and the degree of motor completeness.
The diagram below highlights the overall reduction in lung volumes following SCI, particularly at a cervical NLI.
These changes commonly reflect a restrictive lung pattern.
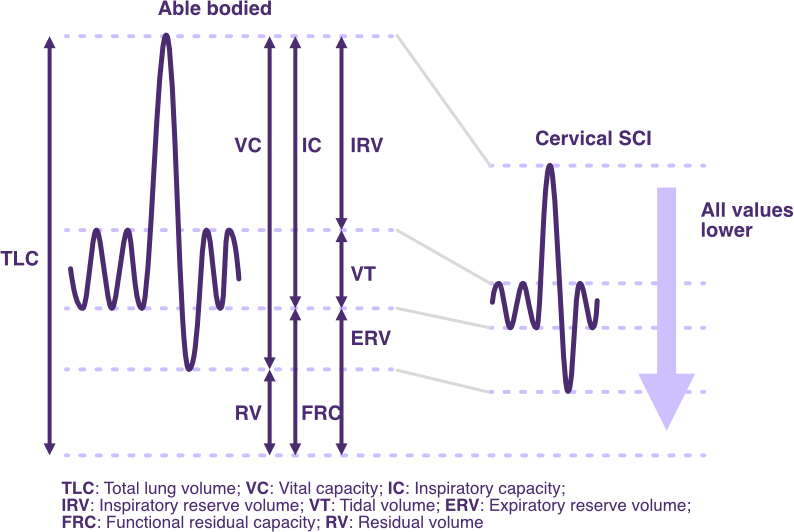
Changes in spirometry lung volumes and capacities following SCI
Adapted from SCIRE Professional
For a full description of spirometry measures, refer to Normal respiratory function: Measures of air volume and flow rate.
Priority assessments
During the acute management phase, spirometry may need to be limited to manage the risk of respiratory fatigue.
Priority spirometry measures to assess are:
INSPIRATORY
- Maximal Inspiratory Pressure (MIP) if possible
- Sniff Nasal Inspiratory Pressure (SNIP) is possible
EXPIRATORY
- Forced Expiratory Volume (FEV1) and Forced Vital Capacity (FVC)
- Unassisted Peak Cough Flow (PCFunassisted)
- Maximal Expiratory Pressure (MEP) if possible.
Devices and interfaces
Ventilators and respiratory devices such as a mechanical insufflator-exsufflator (MI-E) devices may have some inbuilt spirometry. Measures may not accurately represent absolute values, but they may be helpful to detect a change in respiratory function.
Spirometry devices can vary with respect to laboratory versus clinical utility, degree of calibration, cost and portability. Typical spirometry devices with clinical utility include:
- spirometer
- peak flow meter
- respiratory pressure manometer.
When performing spirometry
- use existing ventilation access (e.g. endotracheal tube, tracheostomy, unvented face mask or mouthpiece)
- use the same device and interface until ventilation access changes (improves repeatability to more accurately capture respiratory improvement or decline)
- use repeatable methods of testing, with clear instructions (that elicit the maximal and correct spirometry result).
When assessing peak cough flow
- use a low-cost peak flow meter has been shown to provide acceptable—although slightly underestimated readings—in comparison to high-cost spirometry devices; is more accurate when assessing PCF > 270L/min
- use an unvented face (oronasal) mask—rather than mouthpiece— will allow a more normal coughing action and accurate measure. If a mouthpiece is used, consider use of a nose clip as well.
Positioning
In the acute phase, complete spirometry in the supine position to optimise diaphragm function and to more specifically assess diaphragm function.
When medically stable and able to tolerate sitting, repeat spirometry in the sitting position, to evaluate the effect of sitting (+/- abdominal binder) on diaphragm function.
Spirometry trends
Cervical SCI: VC may be reduced to 20–60% of predicted values.
Thoracic SCI: VC may be reduced to 60–90% of predicted values.
Respiratory pattern: FEV1 is typically reduced in proportion to FVC, consistent with a restrictive lung pattern (with the FEV1/FVC ratio >0.7).
However, this pattern can become mixed due to any pre-existing chronic airway diseases or autonomic nervous system (ANS) disruption—such as bronchospasm or increased sputum production—which causes obstruction (FEV1/FVC ratio <0.7).
Impact of time: Respiratory dysfunction usually improves over time as spinal shock resolves, along with the positive effects of neural plasticity and rehabilitation. Factors contributing to this improvement were discussed earlier.
Spirometry indicators
Spirometry predictive values for a person with SCI are dependent on many factors such as sex, age, height, weight and ethnicity, so the following is only a guide.
Spirometry should be used to identify respiratory function trends to inform management, rather than just capturing absolute values in isolation. Results should be considered in the context of a holistic assessment, including the potential risks of acute or chronic respiratory complications.
The following spirometry values are indicative only, reflecting evidence-based values reported in the literature relevant to acute neuromuscular disorder management, which may have relevance for SCI. These should not be used in isolation, but in the context of all assessment findings—including O2 and CO2 measures—to support clinical reasoning.
FVC < 50% of predicted indicates significant reduction in lung volumes and respiratory muscle strength: there is a high risk of developing hypoventilation and retained secretions—use of lung volume augmentation and cough augmentation is indicated.
PCF < 270 L/min indicates reduction in lung volumes and respiratory muscle strength: there is a high risk of developing retained secretions—use of lung volume augmentation, as well as manual assist cough is indicated.
Use of a mechanical assisted cough device may be preferable if there is a high risk of respiratory fatigue and further respiratory deterioration.
PCF < 160 L/min indicates significant reduction in lung volumes and respiratory muscle strength: there is a high risk of developing retained secretions, even with a manual assisted cough—use of lung volume augmentation as well as mechanical assisted is indicated.
If tolerated, a manual assisted cough may be added in addition to this.
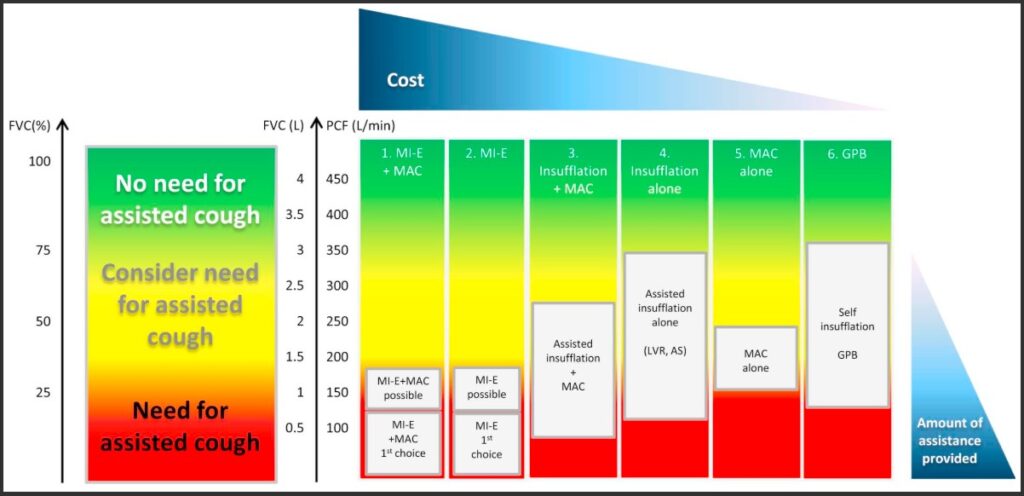
Graph of spirometry indicators for lung volume augmentation and cough augmentation in neuromuscular disorders
Toussaint, M., Chatwin, M., González, J., Berlowitz, D. J., & ENMC Respiratory Therapy Consortium. (2018). 228th ENMC International Workshop: Airway clearance techniques in neuromuscular disorders, Naarden, The Netherlands, 3–5 March, 2017. Neuromuscular Disorders, 28(3), 289–298. https://dhttps://doi.org/10.https://www.nmd-journal.com/article/S0960-8966(17)30588-6/fulltext#f00101016/j.nmd.2017.10.008
MEP < 60 cmH20 indicates significant reduction in expiratory muscle strength: cough is ineffective and there is a high risk of developing retained secretions, even with a manual assisted cough—lung volume augmentation as well as mechanical +/- manual assisted cough is indicated.
FVC < 30% of predicted indicates very significant reduction in lung volumes and respiratory muscle strength: there is a high risk of hypoventilation and in the context of respiratory fatigue could progress to respiratory failure—ventilation support is indicated.
MIP < -40 cmH20 indicates significant reduction in inspiratory muscle strength and likely increased work of breathing: there is a high risk of impending respiratory fatigue and failure—ventilation support is indicated.
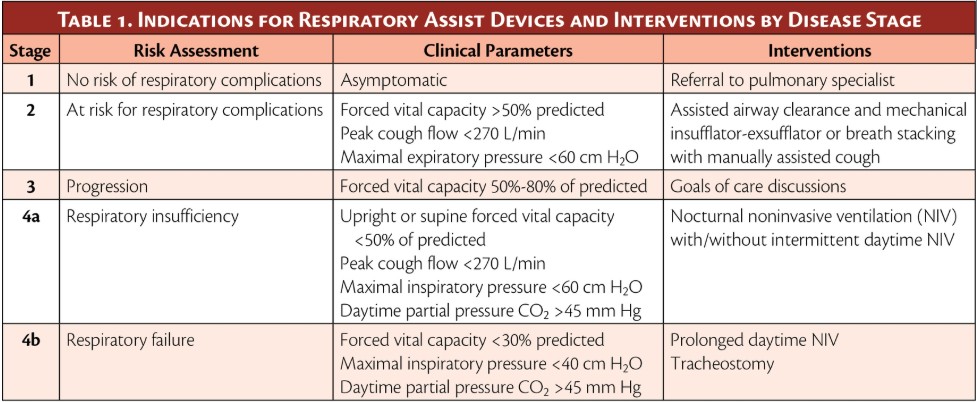
Table of spirometry indicators for interventions at progressive respiratory stages in neuromuscular disorders
Metjian, H. M., & Provost, K. (2022, July–August). Respiratory therapy for neuromuscular disorders. Practical Neurology: Cover Focus. Retrieved from https://practicalneurology.com/articles/2022-july-aug/respiratory-therapy-for-neuromuscular-disorders
Respiratory muscle assessment
Respiratory muscle assessment
The diaphragm, intercostal, and abdominal muscles are the primary respiratory muscles to assess, along with a range or accessory muscles. For more information, refer to Respiratory muscles.
A holistic assessment of respiratory muscle function should involve a neurological assessment, breathing pattern analysis, inspiratory and expiratory muscle palpation, along with a review of the work of breathing.
Neurological assessment
NLI above T12 → increasing paralysis of lower intercostal and lower abdominal muscles.
NLI above T6 → increasing paralysis of intercostal muscles, with full paralysis of all abdominal muscles.
NLI at C5 or above → increasing paralysis of the diaphragm and some accessory muscles, with full paralysis of all intercostal and abdominal muscles.
NLI at C2 or above → increasing paralysis of respiratory accessory muscles, with full paralysis of all intercostal, abdominal and diaphragm muscles i.e. nil spontaneous breathing.
For information, refer to Respiratory Predictive Factors.
Breathing pattern analysis
Altered breathing patterns may be caused by factors such as pain inhibition, altered consciousness etc. However, it may also indicate impaired respiratory muscle function, while highlighting the risk of hypoventilation and respiratory fatigue.
Paradoxical breathing pattern
Palpate the anterior rib cage while observing the intercostal spaces and abdominal rise during quiet and forced inspiration.
• Anterior chest wall collapse and intercostal recessions with excessive abdominal rise typifies paradoxical breathing, including flaccid muscle paralysis of the intercostal and abdominal muscles.
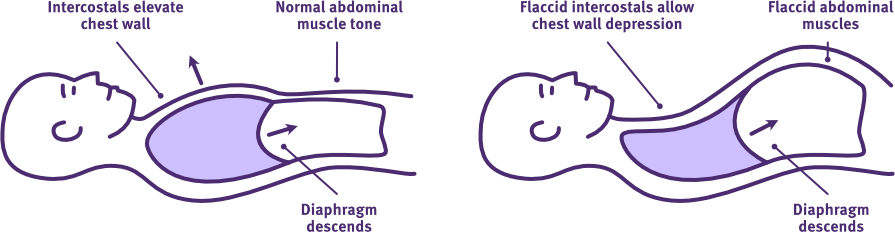
The normal versus paradoxical breathing pattern following acute high-level SCI
Adapted from Thoracic Key
For more information, see Respiratory Changes: Paradoxical breathing.
Inspiratory muscle assessment
Diaphragm palpation
Qualitative diaphragm movement can be assessed when viewing the patient in the supine position from the foot end of the bed to assess symmetry on quiet and deep inspiration.
Bilaterally palpate the upper abdomen during quiet and deep inspiration.
• Poor abdominal rise may indicate diaphragm weakness or abdominal restriction (e.g. paralytic ileus).
• Excessive abdominal rise is a feature of paradoxical breathing, indicating flaccid muscle paralysis of the abdominal muscles and loss of positioning support of the diaphragm.
• Asymmetry of abdominal rise may suggest hemidiaphragm dysfunction.
Intercostal muscle palpation
Bilaterally palpate the lower lateral ribcage during quiet and deep inspiration.
• Poor expansion may indicate intercostal weakness.
• Collapse of the anterior chest wall and intercostal recessions are features of paradoxical breathing, indicating flaccid muscle paralysis of the intercostal muscles.
Accessory muscle palpation
Palpate the scalene, sternocleidomastoid, and upper trapezius muscles during quiet and deep inspiration.
• Significant activity during quiet breathing indicates increased work of breathing, risk of respiratory fatigue, and potential for hypoventilation.
Expiratory muscle assessment
Abdominal muscle palpation
Palpate the abdomen bilaterally during forced expiration and cough versus a huff.
• Low-volume exhalation or cough indicates abdominal muscle weakness or paralysis (in addition to reduced inspiratory capacity). Determine if cough is effective, noting that ineffectiveness may relate to expiratory muscle weakness and/or fatigue, but also mucous viscosity.
• Reduced voice projection may indicate loss of transdiaphragmatic pressure generation from significant abdominal muscle paralysis (in addition to reduced inspiratory capacity).
Work of breathing review
Work of breathing review
The presence of any increased work of breathing, along with any progressive worsening, heightens the risk for respiratory fatigue and possibly respiratory failure. Any of the following signs and symptoms constitutes an increased work of breathing:
- paradoxical breathing
- increased airway/inspiratory resistance e.g bronchospasm, atelectasis, sputum retention
- any compensatory responses to declining ventilation e.g. increased respiratory rate, recruitment of accessory muscles in speech or quiet breathing
- activities related to personal and acute medical care
For more information, refer to Work of breathing.
Sputum and saliva clearance check
Sputum and saliva clearance check
In addition to mobilising sputum from peripheral airways into the mouth, it is also important to determine if there are particular care needs related to sputum and/or saliva clearance from the mouth. This may be due to reduced consciousness, spinal precautions, impaired upper limb function or dysphagia etc.
Ineffective sputum and/or saliva clearance from the mouth can not only be distressing for the person with SCI, but it can also become a risk for aspiration or choking. This risk may be compounded by other factors such as reduced consciousness, pain etc.
Sputum and saliva productivity
Identify whether the person’s cough and huff are dry or moist, and also whether the person’s mouth is similarly dry or moist.
Note the volume of sputum and saliva production, as well as colour and thickness; also, whether both are being effectively cleared from the mouth, either by swallowing or other strategies.
The risk of vomiting or reflux should also be considered in the context of managing oral clearance.
Oral care plan
Observation and treatment notes, along with swallowing assessments should inform an oral care plan and may include:
- positioning in side-lying to reduce risk of aspiration and improve clearance
- regular mouth suctioning and hygiene via nursing care, plus yankeur suction unit within the person with SCI’s reach
- speech pathology referral for swallowing and oromotor assessment
- appropriate medication to reduce sputum and saliva production, or vomiting and reflux
- anti-cholinergic medications
- antibiotic therapy
- antiemetic medications
- appropriate humidification, hydration and mucolytic agents to aid sputum clearance
- physiotherapy referral for additional treatment to aid sputum clearance
- regular monitoring including auscultation, to detect any signs of aspiration, desaturation or respiratory distress.
Swallowing and voice assessment
Swallowing and voice assessment
Dysphagia
A person with high-level cervical SCI should have an early swallow and voice assessment, especially following extubation or cervical spinal surgery (e.g. anterior cervical discectomy and fusion).
For more information on swallowing dysfunction, refer to Dysphagia.
Voice and phonation
A review of a person with SCI’s voice and phonation may highlight the risk of respiratory fatigue, as well as highlight the need for additional nurse call and communication systems.
When spirometry is not immediately available following an acute SCI, timed sustained phonation also offers a simple method to estimate lung capacity, flow rates, and respiratory muscle strength.
A common approach involves asking the individual to sustain the vowel sound “a” at a normal pitch and volume. Typical sustained phonation times for adults range from 15 to 35 seconds, with males generally achieving longer durations.
During the rehabilitation phase following SCI, voice and phonation tasks can serve dual purposes:
• as a functional assessment of respiratory support needed for speech to promote communication (e.g. ventilation settings, lung volume augmentation techniques)
• as an intervention for respiratory muscle training.
For example, following a maximal inhalation or air stacking using a lung volume recruitment (LVR) bag, the number of words spoken in one breath can be counted to assess augmented lung volume for expiratory control.
Medical investigations
Medical investigations
A range of medical investigations may be used to assess changes in respiratory function and identify the onset of secondary complications following SCI. These investigations may include:
Blood pathology
• arterial blood gas analysis to assess gas exchange and ventilation status
• screening for infection and electrolyte imbalances.
Hypoventilation develops when ventilation is inadequate, resulting in insufficient removal of CO2 from the blood and potentially insufficient O2 intake.
If sustained and significant, hypoventilation is associated with
- hypercapnia, which is a build-up of CO2 in the blood (PaCO₂ to levels >45 mmHg)
- hypoxaemia, which is a deficiency of especially O2 in the blood, which worsens during sleep (PaO₂ <60 mmHg).
Sputum pathology
- screening for infection.
Radiological lung imaging
• chest x-ray to detect any lung consolidation or collapse as well as check diaphragm positioning, including any hemidiaphragm abnormalities
• diaphragm ultrasound to evaluate diaphragm function, although it is less effective than spirometry for assessing performance and quantifying improvements
• computed tomography pulmonary angiogram (CTPA) to investigate suspected pulmonary embolism.
Other respiratory function tests
• comprehensive spirometry (e.g. plethysmography, flow-volume loops)
• investigations for sleep-disordered breathing (e.g. overnight oximetry, polysomnography).
Ongoing respiratory monitoring
When a person with an acute, high-level SCI who is spontaneously breathing is admitted to a hospital ward, there is a risk of respiratory deterioration.
This applies not only to the initial hospital admission following SCI, but also during re-admissions to hospital for management of acute events or secondary conditions.
Transfers from intensive care units and/or recent changes in respiratory management—for example extubation or decannulation—are associated with a high risk of respiratory deterioration.
General medical and physiotherapy supports alone are typically insufficient.
Specialist respiratory or intensive care supports are also needed to provide best-practice management, establishing a clear clinical pathway for
- timely review and regular monitoring and
- rapid access to additional medical and ventilatory support if required.
If further specialist SCI advice is required for Queensland patients, contact QSCIS.
For people with a new SCI – contact QuickStart.
For people with SCI readmitted to hospital – contact SPOT.
For urgent assistance after-hours, contact the SIU SMO or registrar on-call via PAH switch 07 3176 2111.
Deterioration
Ongoing monitoring by the multi-disciplinary team is very important and facilitates early detection of any signs of deterioration in respiratory function.
Signs and symptoms of respiratory deterioration may include:
- bluish skin discolouration (cyanosis)
- drowsiness and reduced alertness
- reduced capacity for speech or slurred speech
- arrhythmia, including tachycardia (autonomic nervous system (ANS) disruption may limit this response)
- shortness of breath (dyspnoea) and an increased respiratory rate
- reduced air entry and added sounds on auscultation
- decreasing oxygen (O₂) and increasing carbon dioxide (CO₂) levels on pulse oximetry and blood gas analysis
- abnormal breathing patterns, including paradoxical breathing
- increased work of breathing
- decreased ability to co-operate with coughing
- increased sputum production and/or change in sputum colour
- decline in spirometry.
Reasons for deterioration:
- poor positioning
- insufficient ventilation support, lung volume augmentation and secretion management
- development of respiratory/medical complications
- combination of above contributing to respiratory fatigue and failure.
Reasons for improvement:
- stabilised cardiovascular function
- early and optimal ventilation support
- prevention and treatment of secondary respiratory complications
- resolution of spinal shock, leading to a transition from flaccid to spastic tone of respiratory muscles, resulting in:
- reduced abdominal and chest wall compliance, improving diaphragm function
- reduced breathing effort, especially when sitting upright
- reduction of inflammation and oedema around the spinal cord and secondary injury sites (e.g. chest wall), along with neural plasticity
- general muscular strengthening and training, improving recruitment of the diaphragm and other respiratory muscles from neural plasticity and rehabilitation interventions
- improved cardiopulmonary fitness through mobilisation, rehabilitation and engagement in activities of daily living.
Respiratory impairment in SCI (2025)
PM&R KnowledgeNow
Spinal cord injury guidelines: Guidelines for respiratory management following spinal cord injury (2021)
University of Arkansas for Medical Sciences
Respiratory management following spinal cord injury: A clinical practice guideline for health-care professionals (2005)
Consortium of Spinal Cord Medicine Clinical Practise Guidelines
eLearn SCI— physiotherapists—respiratory: assessing and treating module
eLearn SCI.org
Respiratory education modules and YouTube channel
Canadian Alternatives in Noninvasive Ventilation (CANVent)
Spirometry and peak cough flow instructions
SCIRE Professional
How to perform a peak cough flow test at home
University Hospital Southampton NHS Foundation Trust
Berlowitz, D. J., Wadsworth, B., & Ross, J. (2016). Respiratory problems and management in people with spinal cord injury. Breathe (Sheff), 12(4), 328–340. https://doi.org/10.1183/20734735.012616
Brennan, M., McDonnell, M. J., Duignan, N., Gargoum, F., & Rutherford, R. M. (2022). The use of cough peak flow in the assessment of respiratory function in clinical practice: A narrative literature review. Respiratory Medicine, 193, 106740. https://doi.org/10.1016/j.rmed.2022.106740
Chatwin, M., Toussaint, M., Gonçalves, M. R., Sheers, N., Mellies, U., Gonzales-Bermejo, J., Sancho, J., Fauroux, B., Andersen, T., Hov, B., Nygren-Bonnier, M., Lacombe, M., Pernet, K., Kampelmacher, M., Devaux, C., Kinnett, K., Sheehan, D., Rao, F., Villanova, M., Berlowitz, D., & Morrow, B. M. (2018). Airway clearance techniques in neuromuscular disorders: A state of the art review. Respiratory Medicine, 136, 98–110. https://doi.org/10.1016/j.rmed.2018.01.012
Chen, C. F., Lien, I. N., & Wu, M. C. (1990). Respiratory function in patients with spinal cord injuries: Effects of posture. Paraplegia, 28(2), 81–86. https://www.nature.com/articles/sc199010.pdf
Galeiras Vázquez, R., Rascado Sedes, P., Mourelo Fariña, M., Montoto Marqués, A., & Ferreiro Velasco, M. E. (2013). Respiratory management in the patient with spinal cord injury. BioMed Research International, 2013, Article 168757. https://doi.org/10.1155/2013/168757
Harvey, L. A. (2008). Management of spinal cord injuries: A guide for physiotherapists. Churchill Livingstone Elsevier.
Metjian, H. M., & Provost, K. (2022, July–August). Respiratory therapy for neuromuscular disorders. Practical Neurology: Cover Focus. Retrieved from https://practicalneurology.com/articles/2022-july-aug/respiratory-therapy-for-neuromuscular-disorders
Sperandio, E. F., Arantes, R. L., Matheus, A. C., Silva, R. P., Lauria, V. T., Romiti, M., Gagliardi, A. R., & Dourado, V. Z. (2016). Restrictive pattern on spirometry: Association with cardiovascular risk and level of physical activity in asymptomatic adults. Jornal Brasileiro de Pneumologia, 42(1), 22–28. https://doi.org/10.1590/S1806-37562016000000030
Toussaint, M., Chatwin, M., González, J., Berlowitz, D. J., & ENMC Respiratory Therapy Consortium. (2018). 228th ENMC International Workshop: Airway clearance techniques in neuromuscular disorders, Naarden, The Netherlands, 3–5 March, 2017. Neuromuscular Disorders, 28(3), 289–298. https://dhttps://doi.org/10.https://www.nmd-journal.com/article/S0960-8966(17)30588-6/fulltext#f00101016/j.nmd.2017.10.008
Willis, L. D. (2023). Cough peak flow assessment: Is there more to the story? Respiratory Care, 68(4), 553–555. https://doi.org/10.4187/respcare.10900
Winslow, C., Bode, R. K., Felten, D., Chen, D., & Meyer, P. R., Jr. (2002). The impact of respiratory complications upon length of stay and hospital costs in acute cervical spine injury. Chest, 121(5), 1548–1554. https://doi.org/10.1378/chest.121.5.1548