Ventilation support
A spinal cord injury (SCI) with a neurological level of injury (NLI) above T12 will result in some degree of respiratory function change. Early assessment will determine predictive factors for respiratory function changes and complications. Frequent monitoring will determine the adequacy of ventilation and sputum clearance, as well as any deterioration in breathing patterns and increase in the work of breathing.
Outcomes will ultimately determine ongoing ventilation and respiratory health needs, depending on the extent of chronic respiratory dysfunction and persistent respiratory risk factors.
Respiratory changes
Respiratory changes
There are numerous aspects to respiratory function change following an acute spinal cord injury (SCI).
These may include:
- respiratory neuromuscular weakness involving the intercostals, abdominals and possibly even the diaphragm
- reduced diaphragm efficiency due to altered chest wall and abdominal muscle tone and compliance
- restricted inspiratory capacity and decreased forced expiratory volumes and flow rates
- impaired sigh, cough and other forced expiratory actions
- atelectasis and sputum retention
- hypoventilation, which may progress to hypoxaemia and hypercapnia
- increased work of breathing due to altered breathing mechanics—especially in sitting
- increased risk of respiratory fatigue and failure, as well as sleep-disordered breathing.
In summary, these changes collectively lead to reduced ventilation and secretion clearance, along with an increased work of breathing.
For an acute, motor-complete NLI at T6 or above, but especially at C5 and above, significant respiratory function changes occur due to neuromuscular weakness and paralysis, compounded by the presence of spinal shock, as well as autonomic nervous system (ANS) disruption. Key respiratory muscles affected include the intercostal, abdominal and diaphragm muscles.
At this time, weakened or paralysed respiratory muscles present with flaccidity and increased compliance due to spinal shock. This affects diaphragm positioning and function, as well as breathing mechanics. Ultimately, this results in reduced ventilation and secretion clearance. Paradoxical breathing can also develop, which is very inefficient and increases the work of breathing.
For more information on respiratory function changes following SCI, refer to Respiratory changes.
Without adequate and timely intervention, the combination of hypoxaemia, hypercapnia and respiratory fatigue is a key precursor to the onset of respiratory failure. In addition to this, chronic hypoventilation also contributes to sleep-disordered breathing, compounded by any other obstructive and central factors.
During the acute management phase, the following key interventions should be implemented to optimise respiratory management:
• ventilation support
• lung volume augmentation
• secretion management.
These interventions are complementary and should be tailored to the person with SCI’s individual needs. While both ventilation and lung volume augmentation interventions may involve similar elements—such as patient positioning and the use of positive pressure breathing—their roles and application differ. The distinction is defined as follows.
Ventilation support includes use of positioning and mechanical ventilator devices—often continuously—to normalise gas exchange, stabilise the airway, and reduce the overall work of breathing.
Lung volume augmentation includes a range of respiratory techniques and devices—typically in short, repeated treatment sessions—to achieve the therapeutic benefits of deep breathing, support secretion clearance, and promote respiratory muscle conditioning.
The role of mechanical ventilation
Following a high-level SCI, significant hypoventilation may develop due to respiratory muscle weakness and paralysis, affecting the ability to generate normal tidal volumes and access inspiratory reserve volumes. Expiratory volumes and flow rates are also reduced, impacting secretion clearance. Altered breathing patterns develop, as well an increased work of breathing. The combined outcome of hypoventilation is an increased risk of sputum retention, respiratory fatigue and ultimately respiratory failure.
Supine positioning is an important “first-response” and management consideration. This is not only because spinal precautions may be required, but because supine positioning will complement mechanical ventilation to improve respiratory function.
Specifically, when spinal shock is present, supine positioning helps counteract the adverse effects of abdominal muscle weakness, flaccidity and increased compliance on diaphragm positioning and function. Similarly, mechanical ventilation provides positive pressure breathing which addresses the impact of intercostal muscle weakness, flaccidity and increased chest wall compliance. Together, these interventions are complementary, as both correct aspects of the paradoxical breathing pattern and reduce the work of breathing.
The primary goals of mechanical ventilation are to:
- establish a patent airway, for controlling ventilation and actioning secretion clearance
- provide support to weakened or paralysed respiratory muscles, especially the diaphragm
- using positive pressure breathing to correct a paradoxical breathing pattern and drive oxygen into the lungs
- improving tidal volumes and minute ventilation, addressing hypoventilation
- reversing atelectasis, improving collateral ventilation and gas exchange of O₂ and CO₂
- stimulating surfactant production, improving alveolar compliance and reducing airway resistance
- managing CO₂ retention and the risk of hypercapnia progressing to respiratory acidosis
- managing secretion retention and the risk of pneumonia
- reducing the work of breathing and the risk of respiratory fatigue
- manage the multifactorial risk of respiratory failure (typically occurs between 1-5 days following high-level cervical SCI).
Types of ventilation support
While supplemental oxygen—particularly high flow oxygen—may improve alveolar ventilation and provide a degree of positive airway pressure, typically this will be an insufficient form of ventilation support following a high-level SCI. Rather, mechanical ventilation is typically required for a time, to compensate for the impact of respiratory muscle weakness or paralysis on ventilation.
Mechanical ventilation infers that a ventilator device is used—as well as a circuit and an airway interface—to provide ventilation support.
Invasive ventilators require either an endotracheal tube or tracheostomy interface, while non-invasive ventilation (NIV) devices use a mask or similar interface applied to the face.
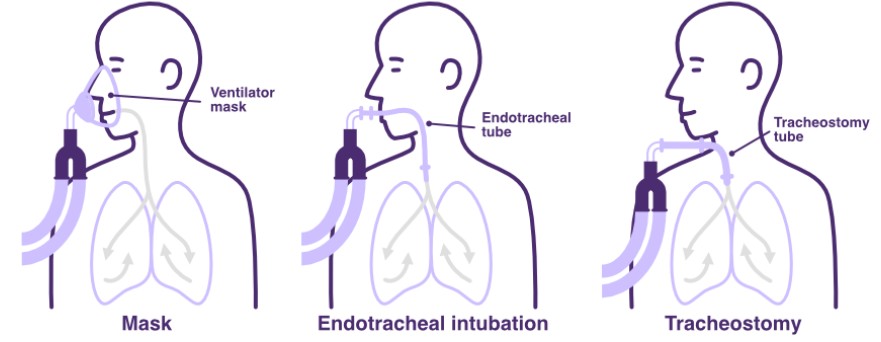
Methods of ventilation support
Adapted from SCIRE Professional
Titrated oxygen allows regulation of FiO₂ (selected blend of room air and oxygen), while humidification again improves heat and moisture to the upper airway to improve the mucociliary transport system, as well as reduce mucous viscosity and tenacity.
Specialist units with expertise in SCI management and NIV, may be able to implement nuanced interventions when ventilation support is required for higher NLI. Generalist units will typically need to adopt a more conservative approach, using invasive ventilation and early insertion of a tracheostomy when there is a high risk of respiratory fatigue and failure in the acute phase post-injury.
Invasive ventilation
Invasive ventilation bypasses the upper airway to provide stable airway access for ventilation control and secretion management.
It is therefore typically the most effective means of ventilation support for managing the risk of respiratory fatigue and failure, especially in the presence of an elevated sputum load. However, this requires careful management: specific titration of ventilator settings to optimise ventilation, synchronise breaths with any spontaneous respiratory effort and eventually stage a process of weaning and extubation.
Endotracheal intubation particularly limits independent coughing and speech, as well as the ability to swallow (impairs glottal and epiglottal function). Whereas tracheostomy insertion still provides airway patency, while enabling swallowing and some capacity for expectoration and communication.
Overall, there are no universal, evidence-based guidelines for mechanical ventilation in acute and sub-acute SCI management. While individual intensivists and respiratory physicians will have protocol preferences, general trends in the published literature highlight a range of clinical indicators, management approaches and outcome limitations for invasive mechanical ventilation. These are summarised below.
Clinical indicators
A range of physiological and clinical markers can indicate the need for invasive ventilation to address the onset of respiratory fatigue and/or failure.
Potential physiological markers include:
- impaired alveolar ventilation
- SpO₂ <90 % on supplemental oxygen
- PaO₂ <60 mmHg on room air
- PaCO₂ >45 mmHg and/or pH <7.35 on room air
- declining spirometry and measures of ventilation, including reduced minute ventilation
- respiratory rate (RR) >30 breaths/min or <8 breaths/min indicating either respiratory stress or disruption to central control
- maximal inspiratory pressure (MIP) <-40 cmH20 indicating significantly reduced inspiratory muscle strength and a high risk of developing respiratory fatigue
- tidal volume (VT) <5 mL/kg
- forced vital capacity (FVC) <15-20 mL/kg or <30% of predicted
- peak cough flow (PCF) <160 L/min and MEP <60 cmH20 (spontaneous breathing) indicating a high risk of retained secretions and associated respiratory complications
- radiological evidence of significant hemi-diaphragm dysfunction, atelectasis, aspiration or pneumonia.
Potential clinical indicators include:
- a decreased level of consciousness or capacity to maintain a patent airway, including abnormal bulbar function
- a high NLI such as
- an SCI at C1–2, with complete diaphragm, intercostal and abdominal muscle paralysis
- an acute SCI at C3, with significant diaphragm weakness and dysfunction, as well as full intercostal/abdominal paralysis
- an acute SCI at C4–5, with partial diaphragm weakness and dysfunction, as well as full intercostal/abdominal paralysis
- any acute SCI at C6-T6, with full diaphragm muscle activity, but ongoing dysfunction due to full or partial intercostal/abdominal muscle impairment
- an ascending neurological level of cervical or thoracic SCI
- traumatic SCI with significant concomitant injuries or complications, impairing respiratory function
- age >50 years and comorbidities, including chronic pulmonary obstructive disease
- a high sputum load and weakened or absent cough, with ineffective secretion clearance
- paradoxical breathing in the presence of spinal shock and significant ANS dysfunction
- signs of increased work of breathing and the onset of respiratory fatigue
- progression towards or onset of respiratory failure, which typically occurs by day 1–5 post-injury—even with non-invasive ventilation use
Evidence-based SCI management
Current evidence-based SCI management approaches include:
- traumatic SCI at C5 and above, or a high NLI SCI with lung injury, typically require intubation and invasive mechanical ventilation support >80% of the time
- elective intubation is preferable to emergency intubation to avoid further spinal cord damage, related to cervical spine movement during the procedure
- early tracheostomy (≤Day 7) with invasive mechanical ventilation, may be associated with a decrease in duration of mechanical ventilation and ICU/hospital admission; also, a reduced incidence of ventilator-associated pneumonia (VAP), but not mortality
- full-assist or partial control modes of ventilation may promote development of ventilator-induced diaphragmatic dysfunction (VIDD) in sedated or paralysed patients within 18 hours; this is due to inactivity/reduced load on the diaphragm, permitting the onset of atrophy
- acute SCI patients with lung injury and at risk of adult respiratory distress syndrome (ARDS), will require lung-protective ventilation tidal volumes, to manage the risk of further barotrauma
- sub-acute SCI patients, are generally not at risk of ARDS and without higher ventilation tidal volumes, may be more prone to atelectasis and failure to wean from mechanical ventilation
- very high tidal volume ventilation >20-25 mL/kg may increase the risk of VAP and other adverse respiratory outcomes e.g. ventilator dependence
- acute and sub-acute SCI with nil lung injury, benefit from high ventilation tidal volumes up to 15 mL/kg, with individualised levels of positive end expiratory pressure (PEEP) 0-7 cmH20 to prevent atelectasis
- when transitioning to a high tidal volume ventilation, use of a graduated approach may be advisable to daily increase tidal volume by 100 mL and flow rate by 10 L/min, until
- peak airway pressures reach 30-40 cmH20
- arterial blood gases and/or end-tidal CO₂ (if a low PaCO₂ (respiratory alkalosis), add dead space to the ventilator circuit to maintain PaCO₂ within the normal range (35–45 mmHg))
- chest radiographs confirm resolution of atelectasis
- higher ventilation tidal volumes are often preferred by people with SCI to
- decrease the work of breathing and the sensation of dyspnoea
- augment tracheostomy speech (cuffless/fenestrated/deflated cuff), including alterations to ventilator settings such as increased PEEP and inspiratory time.
Negative outcomes
Potential negative and adverse outcomes include:
- ventilation dependency and slow weaning, including the development of VAP and VIDD
- prolonging ICU and hospital admission
- limiting progression to sitting and engagement in rehabilitation
- invasive ventilation restricting laryngeal function and the capacity for speaking: this has been reported to increase patient frustration, fear, powerlessness and anxiety, as well as the incidence of treatment error
- impaired swallowing: this necessitates alternative feeding and hydration management
- endotracheal intubation and tracheostomy complications such as granulation formation, stomal infection, tracheomalacia, tracheal perforation, stenosis and fistula formation
- laryngeal dysfunction post extubation and decannulation
- full-time and life-long invasive ventilation may be required for an SCI at C1-2, and possibly C3, in the absence of neurological recovery.
Non-invasive ventilation (NIV)
NIV delivers positive-pressure breathing and airway support to support ventilation, without bypassing the upper airway.
It therefore preserves the ability to cough, speak and swallow normally—significantly enhancing comfort and communication. NIV also improves synchrony between the device and user, while avoiding potential complications associated with invasive ventilation such as ventilator-associated pneumonia (VAP), ventilator-induced diaphragmatic dysfunction (VIDD) and laryngeal dysfunction.
NIV devices apply positive airway pressure, so that the pressure outside the lungs exceeds the pressure inside. This pressure gradient drives air into the lungs, improving lung compliance. This has the effect of reducing the respiratory effort to initiate inspiration and therefore, the overall work of breathing. Some forms of NIV also improve tidal volumes, by setting an inspiratory airway pressure (IPAP) which is higher than the end expiratory pressure (EPAP).
In addition to this, NIV helps maintain lung expansion by increasing functional residual capacity—the volume of air remaining in the lungs after tidal exhalation. This residual alveolar air enhances gas exchange and reverses atelectasis.
However, NIV does not permit any direct airway access for suctioning.
Again, there are no universal, evidence-based guidelines for the use of NIV in the acute and sub-acute management of SCI. While practice varies between clinicians, the literature does highlight some general trends regarding clinical indicators, considerations and the outcome limitations for NIV.
Clinical indicators
To be appropriate for NIV, a person with a SCI must be conscious and have capacity to maintain a patent airway, including normal bulbar function.
A range of clinical markers can indicate the need for NIV to manage the onset of respiratory fatigue and/or failure.
Potential clinical indicators include:
- a lower NLI and motor-incomplete injury
- less significant concomitant injuries or complications, impairing respiratory function
- age <50 years and less significant comorbidities
- a low sputum load and some capacity for secretion clearance
- reduced minute ventilation, where only tidal volume needs some improvement
- impaired alveolar ventilation, where only functional residual capacity needs enhancement
- some signs of increased work of breathing over 24-hour period, but no immediate risk of respiratory fatigue
- an appropriate goal to permit independent ventilation and some speech
- a need to avoid long-term complications associated with invasive ventilation e.g. VAP, VIDD, laryngeal dysfunction
- weaning from invasive ventilation, when ongoing ventilatory support is still required
- short and long-term management of sleep-disordered breathing.
Other considerations
NIV requires an expiratory valve/leak port in the interface or circuit to prevent CO2 rebreathing.
Considerable time is often required to optimise the interface fit. This is to minimise unwanted air leakage for successful ventilation support, while also maximising skin care, comfort and user compliance. Options to trial may include nostril or nasal pillows, along with face masks and mouthpieces.
Non-invasive ventilation is typically prescribed by an intensivist or a sleep/respiratory physician. An experienced physiotherapist or nurse may also lead its implementation, in consultation with the medical team.
Beyond the acute management phase, a person with SCI may be prescribed NIV for use in the community. Ongoing support and review is useful to assist user compliance, by problem-solving technical issues, as well as adjusting titration as respiratory function changes e.g. ageing.
Negative outcomes
During the acute phase of management, the appropriateness of the selected NIV mode and parameters should be evidenced by improved ventilation measures, preferably within 1-2 hours of commencement.
Potential negative and adverse outcomes include:
- frequent nursing cares and therapy treatments if sputum load increases, because it does not provide direct airway access
- inadequate improvements in ventilation and relief from the work of breathing, with worsening hypercapnia and respiratory acidosis
- complications when using supplemental oxygen, due to inadequate monitoring and titration e.g. may mask worsening respiratory status
- complications such as gastric distension, aspiration
- difficulty achieving user tolerance and compliance with the interface and ventilator settings
- trial process can be time and staff intensive, as well as distressing for the person with an SCI.
NIV support
Adapted from SCIRE Professional
The following factors may prohibit the safe or effective use of NIV:
- reduced airway protection, including bulbar dysfunction
- any increased aspiration risk
- reduced consciousness, comprehension and cooperation
- severe facial injuries
- poorly controlled intracranial pressures
- chest wall, thoracic or abdominal trauma or surgery (e.g. undrained pneumothorax, tracheoesophageal fistula, paralytic ileus)
- haemodynamic instability or trauma, including pulmonary embolism
- poor ventilatory status
- high sputum load and inability to maintain effective coughing over a prolonged period.
During acute management, NIV:
- should not delay intubation when invasive ventilation is indicated
- needs to be trialled appropriately and titrated optimally
- must only be introduced after establishing baseline measures of ventilation etc.
- should demonstrate efficacy within 1-2 hours of commencement
- requires frequent and responsive monitoring, along with adequate staffing and alarm settings
- benefits from clearly documented risk management and action plans
More information on the best-practice introduction of NIV for the management of acute respiratory failure is here.
Types of non-invasive ventilation
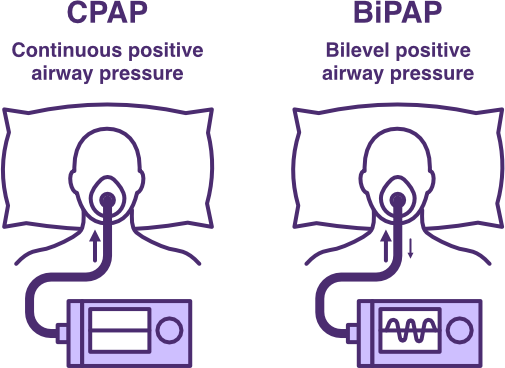
Comparison of CPAP and BiPAP Ventilation
Adapted from SCIRE Professional
Continuous positive airway pressure (CPAP) ventilation
Continuous positive airway pressure (CPAP) ventilation
Continuous positive airway pressure (CPAP) ventilation provides a low level of continuous positive airway pressure through inspiration and expiration.
Overall, CPAP:
- provides continuous positive pressure during both inspiration and expiration
- effectively supports airway patency by
- preventing lower airway collapse and promoting smooth muscle airway relaxation via prostaglandin release
- facilitating collateral ventilation to reduce atelectasis and improve alveolar volume and oxygenation
- increasing functional residual capacity, lung compliance and gas pressure behind secretions
- reduces the overall work of breathing, provided positive pressure is adequate
- assists in the management of
- pulmonary oedema
- weaning process following invasive ventilation
- obstructive sleep apnoea, associated with sleep-disordered breathing.
Typically, start with a positive-end expiratory pressure (PEEP) at 10% of the person’s body weight e.g. between 5–10 cmH₂O.
Bilevel positive airway pressure (BiPAP) ventilation
Bilevel positive airway pressure (BiPAP) ventilation
Bi-level positive airway pressure (BiPAP) ventilation delivers two levels of positive airway pressure across inspiration and expiration.
Overall, BiPAP:
- establishes a pressure gradient between inspiration and expiration
- augmenting tidal volumes
- generating a more effective cough to manage secretion retention
- reducing associated risks of atelectasis and pneumonia
- improving minute ventilation
- delivering pressure support to the lungs
- improving alveolar volume and oxygenation, while also managing hypercapnia
- reducing the overall work of breathing, especially inspiratory effort; this reduces the risk of respiratory fatigue and may delay or prevent intubation
- augmenting tidal volumes
- assists in the management of
- hypoventilation and early progression towards respiratory fatigue and failure
- secretion clearance when there is high risk of respiratory fatigue using more conventional interventions
- weaning high-risk users from invasive ventilation and intubation
- supporting mobilisation and developing exercise tolerance following the acute management phase.
In practice, the inspiratory positive airway pressure (IPAP) is normally set above the expiratory positive airway pressure (EPAP) to increase tidal volume; the greater the difference between IPAP and EPAP, the greater the augmented tidal volume. There may also be other settings to consider such as rise and fall time, as well as sensitivity with respect to triggering a breath or cycle of breaths.
BiPAP devices can offer a range of modes, including:
- Spontaneous (S) mode, the ventilator supports the user’s own breaths
- For example, start with an IPAP of 10 to 15 cmH2O, with an EPAP of 4-5 cmH2O; then increase IPAP in 1cmH2O increments, until maximum tolerance or target tidal volume of 6-8mL/kg ideal body weight is achieved (an IPAP of 12-16cmH2O is often sufficient); slightly higher EPAP may be required e.g. obesity to maintain airway patency, although usually a minimal EPAP is needed; minimise FiO2 to maintain SpO2 88-92%
- Spontaneous/Timed (S/T) mode, the ventilator provides the same support but also includes a backup respiratory rate (BRR) set just below the user’s natural respiratory rate, ensuring a minimum number of breaths
- For example, start with an IPAP of 8cmH2O and an EPAP of 4cmH2O, with a rise time 0.3 seconds, BRR of 12-16 breaths per minute, inspiratory time 1.4 seconds; then increase IPAP in 1cmH2O increments, until maximum tolerance or target tidal volume of 6-8mL/kg ideal body weight is achieved (an IPAP of 12-16cmH2O is often sufficient); slightly higher EPAP may be required e.g. obesity to maintain airway patency, although usually a minimal EPAP is needed; minimise FiO2 to maintain SpO2 88-92%.
Mouthpiece ventilation (MPV)
Mouthpiece ventilation (MPV)
Mouthpiece ventilation (MPV) provides non-invasive ventilation (NIV) on demand, via a mouthpiece. It is typically used in the management of people with degenerative neuromuscular diseases and respiratory dysfunction, who are living in the community. However, it is another ventilation support consideration for a person with SCI, who may require intermittent daytime ventilation support.
MPV is often used during the day to manage the neuromuscular fatigue of inspiration associated with activities of daily living (ADLs), such as sitting, speaking, or coughing. However, when MPV is used during the day, another form of optimised NIV is required for sleeping (providing a degree of neuromuscular rest from the fatigue of spontaneous breathing during the day, while addressing hypoventilation associated with sleep-disordered breathing overnight).
Overall, MPV:
- provides inspiratory ventilation assistance on demand
- to avoid the need for mechanical ventilation (typically when there is a degree of daytime hypercapnia +/- dyspnoea despite optimised nocturnal NIV)
- to support the weaning process for extubation/decannulation
- can be easier to trigger as the negative pressure generated by the mouth during the inspiratory “sip” is much higher than that generated by a deep breath alone
- allows the user to detach from the interface when not required
- minimising claustrophobia
- improving self-image
- reducing the risk of facial pressure sores
- supplements tidal volumes and helps maintain minute ventilation
- supporting the work of breathing when sitting
- permitting air stacking to achieve maximum insufflation capacity (MIC)
- improving independent speech and cough
- reducing care needs associated with frequent need for lung volume and cough augmentation
- providing an alternative means of breathing in case of other ventilator device failure
- reduces the overall work of breathing associated with ADLs
- improves quality of life.
The MPV setup needs to:
- use a suitable ventilator (volume-cycled, pressure/flow triggered)
- provide a
- a single limb circuit
- a filter (to soften the ventilator constant flow at the user)
- a one-way valve (to prevent alarming if the user exhales into the circuit)
- an angled mouthpiece (positioned within reach of the user)
- offer settings to accommodate the user’s oromotor control and achieve a voluntary lip seal
- balance device sensitivity to achieve triggering by a “sip” inspiratory action, but avoid auto-triggering.
The user must:
- have adequate oropharyngeal muscle strength (including control to close their soft palate and seal the nasopharynx, as well as open and their glottis/vocal folds to direct the air into their lungs)
- have sufficient head and neck range of motion to access the mouthpiece repeatedly
- be alert and able to co-operate and communicate.
MPV delivers an on-demand inspiratory volume: either via a single “sip” for normal MPV or via multiple “sips” to perform air stacking. The latter aims to specifically achieve lung volume recruitment (LVR) and considerable optimisation of settings will be important to establish with each user. Achieving a tidal volume of 700-1500 mL for adult patients will typically provide a satisfying to deep breath, while achieving MIC will require a pre-set pressure limit of 45-70 cmH20. Practising LVR is recommended at least 2-3x each day, for up to 5 breaths each time to maintain maximal insufflation capacity. A manual assisted cough may still be required to assist with secretion clearance. This can be determined by comparing peak cough flow (PCFunassisted) following MPV+LVR assisted peak cough flow (PCFassisted) following MPV+LVR+manual cough.
Potential limitations for consideration include:
- a degree of air leak occurs via the nose
- some negative effects can result, such as gastric distension, increased saliva and induced vomiting
- being inappropriate for ventilation support for sleep
- being insufficient ventilation support during periods of acute illness.
A video demonstrating combined use of NIV, including MPV can be found here.
Weaning for extubation and decannulation
Determining readiness to wean from ventilation supports and capacity to manage secretions via a normal airway is very important. This is because both early and delayed weaning and extubation/decannulation increases the incidence of respiratory complications. One recent study reported up to 20% of patients with cervical, motor-complete SCI fail weaning for these reasons.
Clinical guidelines for weaning, extubation and decannulation following SCI are not well established. However, some general indicators and criteria, as well as clinical approaches are suggested below.
Clinical indicators and criteria
General physiological and clinical indicators/criteria include:
- afebrile
- normal chest x-ray
- 48 hours of nil tidal volume ventilation support (with FiO2 <25% and PEEP <5 cmH2O)
- maximal inspiratory pressure (MIP) >24 cmH20 and vital capacity (VC) >1500 mL or >10–15 mL/kg of ideal weight, sustained throughout the day and across several days to demonstrate diaphragm strength and endurance
- peak cough flow (PCF) >160 L/min (or >270 L/min using cough augmentation)
- stable vital signs and medical condition, including during cares
- SpO2 >95% (with FiO2 <25% and PEEP <5 cmH2O)
- blood gases PaO2 approaching 80+ mmHg and PaCO2 approaching 35–45 mmHg
- pH within range of 7.35–7.45
- normal fluid balances
- manageable secretions
- resolution of other injuries or risk factors impacting respiratory function
- no contraindications for NIV or physiotherapy lung volume augmentation and secretion management techniques
- no required surgical or x-ray diagnostic procedures close to extubation that require sedation
- effective pain management
- sufficient alertness and compliance
- adequate swallow function as per feeding plan.
Clinical approach
General clinical approaches include:
- adequate staging
- allowing weaning and decannulation to occur over sufficient period e.g. 7-14 days
- aiming to achieve 2 main stages:
- initially 16 hours ventilator free which represents 1 day period (using NIV as needed)
- then 24-48 hours ventilator free which represents 1-2 day/night cycles (using NIV as needed)
- adequate preparation
- establishing baseline physiological and spirometry measures (MIP, VC, maximal expiratory pressure (MEP) and PCF)
- maximising nutrition and incorporating medications, to increase respiratory muscle mass and build strength/improve diaphragm contractility
- introducing specific lung volume augmentation, including air/breath stacking and inspiratory muscle training (IMT) techniques to
- improve chest wall and lung compliance
- develop respiratory muscle strength
- establish competence and confidence with techniques
- introducing non-invasive secretion management strategies, including mechanical insufflation-exsufflation device and manual assisted coughing to
- clear sputum load
- develop respiratory endurance
- establish competence and confidence with techniques
- introducing early cuff deflations to condition diaphragm and accessory muscles by
- breathing around the cuff, to build tolerance
- using speaking valve, to introduce speech and ventilation demand
- commencing assessment and rehabilitation of swallowing function, to manage any risk of aspiration while improving quality of life
- introducing periodic ventilator-free breathing (PVFB) to achieve a training effect with respiratory muscles
- when staffing levels allow for close monitoring and responsive intervention
- when it is early in the day, following morning cares and a rest period with adequate ventilatory support
- with short, structured trial periods—increasing in frequency and duration: beginning with short 5 min periods, followed by a minimum 2-hour rest for the diaphragm before next session (rest is with unaltered ventilator parameters)
- when positioned in supine for quiet breathing, then during respiratory treatments, then inclined sitting, then sitting out of bed (with an abdominal binder to reduce the work of breathing), then incorporated into functional activities (e.g. being hoisting out of bed or speech sessions)
- once PVFB is well established, gradually
- reducing ventilation tidal volumes during rest periods
- transitioning to pressure support ventilation
- trialling and identifying the most tolerable NIV settings and interfaces
- weaning to daytime NIV (with or without ventilator-free breathing periods)
- gradually introducing overnight NIV
- progressing tracheostomy before full decannulation by
- introducing cuffless tracheostomy/smaller tracheostomy tubes
- optimising stomal seal/use of a cap
- practising secretion clearance to the mouth.
Seek input from experienced SCI clinicians, to guide the weaning process for invasive mechanical ventilation, including extubation and decannulation.
Successful weaning depends on:
- knowledge of respiratory function and ventilation support in the context of SCI
- close monitoring and timely responsiveness
- multidisciplinary collaboration and planning
- documentation of risk management and action plans.
Discharge and community planning
A person with SCI who has ongoing and significant respiratory dysfunction, will require ongoing ventilation and respiratory health supports for community living. Hospital discharge planning processes will need to address funding, care recruitment and training, as well as establish an adequate network of health professional support. A comprehensive process of trialling and scripting will also be important to supply necessary respiratory equipment and consumables.
For further information, refer to Discharge and community planning.
Respiratory impairment in SCI (2025)
PM&R KnowledgeNow
Management of SCI complications—Respiratory (2024)
Canadian Spinal Cord Injury Practice Guideline (Can-SCIP)
Best practices guidelines: Spine injury—Ventilator management in high spinal cord injury (2022)
American College of Surgeons
Respiratory management following spinal cord injury (2022)
SCIRE Professional
Respiratory dysfunction after spinal cord injury (2022)
The Miami Project
Physiotherapy management of people with spinal cord injury (2022)
The Australian and New Zealand Physiotherapy Guidelines for people with SCI
Spinal cord injury guidelines: Guidelines for respiratory management following spinal cord injury (2021)
University of Arkansas for Medical Sciences
Respiratory management following spinal cord injury: A clinical practice guideline for health-care professionals (2005)
Consortium for Spinal Cord Medicine—Clinical Practice Guidelines
Respiratory education modules and YouTube channel
Canadian Alternatives in Non-invasive Ventilation (CANVent)
A comprehensive guide to non-invasive ventilation (NIV) (2024)
Hamilton Medical
Non-invasive ventilation (NIV) for patients with acute respiratory failure: Clinical practice guide (2023)
Agency for Clinical Innovation
Respiratory education videos in non-invasive ventilation (NIV)
Breas
Mechanical ventilation and weaning protocols (2022)
SCIRE Professional
Tracheostomy decannulation (2022)
SCIRE Professional
Abedini, M., Froutan, R., Bagheri Moghaddam, A., & Mazloum, S. R. (2020). Comparison of “cough peak expiratory flow measurement” and “cough strength measurement using the white card test” in extubation success: A randomized controlled trial. Journal of Research in Medical Sciences, 25, Article 52. https://doi.org/10.4103/jrms.JRMS_939_19
Bissett, B., Gosselink, R., & van Haren, F. M. P. (2020). Respiratory muscle rehabilitation in patients with prolonged mechanical ventilation: A targeted approach. Critical Care, 24(1), 103. https://doi.org/10.1186/s13054-020-2783-0
Brown, R., DiMarco, A. F., Hoit, J. D., & Garshick, E. (2006). Respiratory dysfunction and management in spinal cord injury. Respiratory Care, 51(8), 853-870. https://doi.org/10.4187/respircare.05185
Denton, M., & McKinlay, J. (2009). Cervical cord injury and critical care. Continuing Education in Anaesthesia, Critical Care & Pain, 9(3), 82–86. https://doi.org/10.1093/bjaceaccp/mkp013
Fenton, J., Warner, M., Lammertse, D., Charlifue, S., Martinez, L., Dannels-McClure, A., Kreider, S., & Pretz, C. (2016). A comparison of high vs standard tidal volumes in ventilator weaning for individuals with sub-acute spinal cord injuries: a site-specific randomized clinical trial. Spinal Cord, 54(3), 234–238. https://doi.org/10.1038/sc.2015.145
Galeiras Vázquez, R., Rascado Sedes, P., Mourelo Fariña, M., Montoto Marqués, A., & Ferreiro Velasco, M. E. (2013). Respiratory management in the patient with spinal cord injury. BioMed Research International, 2013, Article 168757. https://doi.org/10.1155/2013/168757
Gong, Y., & Sankari, A. (2022, December 11). Noninvasive ventilation. In StatPearls [Internet]. StatPearls Publishing. Retrieved from https://www.ncbi.nlm.nih.gov/books/NBK578188/
Harvey, L. A. (2008). Management of spinal cord injuries: A guide for physiotherapists. Churchill Livingstone Elsevier.
Hendershot, K. A., & O’Phelan, K. H. (2022). Respiratory complications and weaning considerations for patients with spinal cord injuries: A narrative review. Journal of Personalized Medicine, 13(1), 97. https://doi.org/10.3390/jpm13010097
Metjian, H. M., & Provost, K. (2022, July–August). Respiratory therapy for neuromuscular disorders. Practical Neurology. Retrieved from https://practicalneurology.com/articles/2022-july-aug/respiratory-therapy-for-neuromuscular-disorders
Peterson, W., Barbalata, L., Brooks, C. A., Gerhart, K. A., Mellick, D. C., & Whiteneck, G. G. (1999). The effect of tidal volumes on the time to wean persons with high tetraplegia from ventilators. Spinal Cord, 37(4), 284–288. https://doi.org/10.1038/sj.sc.3100818
Pinto, T., Chatwin, M., Banfi, P., Winck, J. C., & Nicolini, A. (2017). Mouthpiece ventilation and complementary techniques in patients with neuromuscular disease: A brief clinical review and update. Chronic Respiratory Disease, 14(2), 187–193. https://doi.org/10.1177/1479972316674411
Reyes, M. R. L., Shaffer, J., Kwong, C., & Gater, D. (2020). A primary care provider’s guide to managing respiratory complications after spinal cord injury. Top Spinal Cord Inj Rehabil, 26(2), 116–124. https://doi.org/10.46292/sci2602-116
Toki, A., Nakamura, T., Nishimura, Y., Sumida, M., & Tajima, F. (2021). Clinical introduction and benefits of non-invasive ventilation for above C3 cervical spinal cord injury. The Journal of Spinal Cord Medicine, 44(1), 70–76. https://doi.org/10.1080/10790268.2019.1644474
Wiles, M. D., Benson, I., Edwards, L., Miller, R., Tait, F., & Wynn-Hebden, A. (2024). Management of acute cervical spinal cord injury in the non-specialist intensive care unit: A narrative review of current evidence. Anaesthesia, 79(2), 193–202. https://doi.org/10.1111/anae.16198
Zakrasek, E., Nielson, J., Kosarchuk, J., & Crew, J. (2017). Pulmonary outcomes following specialized respiratory management for acute cervical spinal cord injury: A retrospective analysis. Spinal Cord, 55(6), 559–565. https://doi.org/10.1038/sc.2017.10